Research & Training
Scientific concept of the SFB
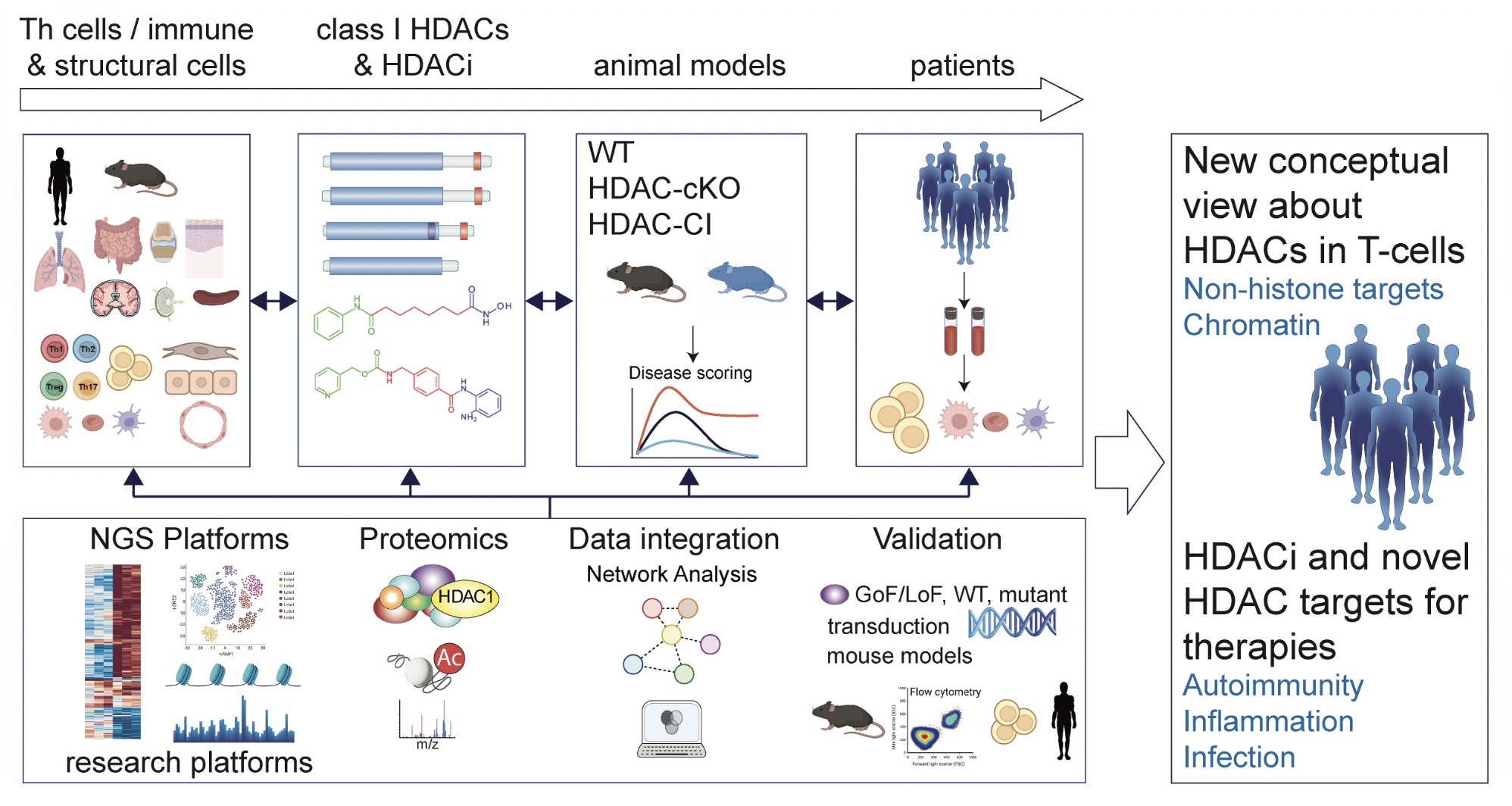
The goal of this SFB is to provide a new conceptional view about HDAC function in T cels and to test essential regulatory roles of reversible lysine acetylation beyond histone modifications and epigenetic gene regulation in Th cells. To achieve these ambitious but feasible aims, our interdisciplinary consortium will focus on Th cells (and their interaction with other immune and structural cells) and a single class of HDACs with strong clinical potential (paradigm class I HDACs), thus providing a high degree of focus, coherence and potential for mechanistic insights as well as translational / biomedical relevance. We will exploit state-of-the art proteomics and next generation sequencing technologies, taking advantage of both animal models and human systems to provide an integrative model of class I HDAC function in T cells in homeostasis as well as the role of HDACs and HDACi for infection and autoimmunity. The expected outcome will fundamentally advance our understanding of how HDACs regulate Th cell lineages beyond the classical epigenetic control of gene expression. Further, the results of the SFB will provide a rationale for the application of isoform-selective HDACi for the treatment of T cell-mediated immune diseases and identify HDAC target genes and target proteins as potential novel drug targets.
SFB research areas
The SFB research program is structured into three major research areas:Area 1
We will study how the ablation/inhibition of HDAC members affects Th differentiation and plasticitiy, T cell-mediated immune diseases and infections with fungal pathogens. We will also study the effect of catalytically inactive HDACs that mimic HDACi treatment on -cell function to distinguish between catalytic and potential structural effects of HDACs. We aim to provide information about the type of T cell-mediated diseases that might be targetable by subclass-specific HDACi in humans.Area 2
We will advance our understanding of how HDACs regulate CD4+ T cell-mediated immunity beyond the classical epigenetic control of gene expression and gene regulatory networks (GRNs). We will address to what extent HDACs exert their function through non-chromatin pathways by regulating non-histone proteins, a key unanswered question in the field. Moreover, class I HDACs operate in multiprotein complexes, but it is unknown whether and how complex composition changes in distinct cell types, upon differentiation or in autoimmunity and infectious diseases, or how the catalytic activity and target specificity of HDACs is regulated at the molecular level in a Th lineage-specific manner.Area 3
We will integrate Th subset-specific datasets including mouse and human NGS and proteome profiles generated under standardized conditions across the SFB consortium. We aim to obtain an integrated view of HDAC-dependent chromatin landscapes, transcriptional changes and acetyl-proteome profiles, as well as the dynamics of HDAC interaction maps in health and disease. Based on these data, we will further validate HDAC targets in human T cells as well as in patients with rheumatoid arthritis (RA), an important autoimmune disease with a major involvement of Th cells.Description of SFB projects
P01 -
Wilfried ELLMEIER: SFB administration and platform coordination.
This project part comprises the scientific and administrative organization of the SFB. A central and essential task of the SFB administration project is, together with the appointed platform coordinators, also the coordination of the research platforms. Next generation sequencing and mass spectrometry experiments will be centrally coordinated, which will also include the standardization and harmonization of sample preparation in order to obtain high-quality NGS and proteomic datasets. These datasets will then be analyzed using the bioinformatics and data integration platform. P01 constitutes the central coordination and interaction hub of the SFB network.Collaborations for P01 within SFB: entire SFB faculty.
P02 – Christoph BOCK: Systems-level analysis of HDAC-dependent Th cell plasticity.
HDAC enzymes have been implicated in multiple aspects of T cell development, but their specific roles remain incompletely understood. In P02, we will take on a systems biology approach to test the hypothesis that HDAC inhibitors enable cell fate control of naïve T cells and selective re-education of committed T cells. We will pursue a comprehensive genomic and systems-level analysis of CD4+ T cell plasticity in response to pharmaceutical and genetic HDAC inhibition, dissecting the impact of HDAC inhibition along the dimensions of histone acetylation, chromatin accessibility and gene expression. Integrative analysis of the resulting datasets will establish bioinformatic regulatory network models for human and mouse T cell differentiation, which provides the foundation for SFB-level data integration with the data obtained in P03, P04, P05, P06 and P08. Finally, the results of the integrative data analysis will be used to generate and prioritize candidate regulatory mechanisms and potential target proteins that will be validated using the broad set of research tools, including genetically engineered mice and pre-clinical disease models that are available in the SFB consortium.This project part is embedded in research area 1 and 3. Collaborations for P02 within SFB: entire SFB faculty.
Information about the project leader & team
P03 – Michael BONELLI: HDACs as targets in systemic autoimmune disease patients.
Our preliminary data reveal that the deletion of certain HDAC family members protects mice against the development of collagen-induced arthritis. We therefore hypothesize that HDACs have a crucial function in the pathogenesis of inflammatory arthritis. We will study effects of selective HDACi on the development of arthritis in a murine T-cell dependent arthritis model, which will also be used for target validation. Experiments are designed to study the effects of HDACi on the chromatin signature and transcriptome of T cell subsets, which will be compared to T cells isolated from patients with Rheumatoid Arthritis. Chromatin profiling will determine enhancer signatures of T cells from RA patients. We will further study whether HDACi can reprogram a pathogenic signature of T cells from RA patients. The combination of murine and human dataset will provide further evidence for the use of HDACi as a new treatment strategy for patients with RA. This project is embedded in research area 1 and research area 3. Collaborations of P03 within SFB: P02, P04, P05, P08, P09.
Information about the project leader & team
P04 – Nicole BOUCHERON: HDACs and Th cell modulation in allergic airway inflammation.
We previously showed that T cell-specific deletion of HDAC1 leads to enhanced Th2 responses, with an exacerbation of inflammation in a murine allergic airway disease model. In addition, we showed that HDAC1 and HDAC2 maintained the identity of CD4+ T cells, implying HDACs as key players in lineage fate commitment and plasticity. Beyond the classification of Th cells in the major subsets known so far, each subset can be adapted or programmed to respond optimally in the context of a particular disease. Failure in this adaptation or programing can lead to allergic conditions. Our main goal in this project is to test the function of HDACs in the modulation/programing of Th cells important for the induction and perpetuation of allergic airway inflammation. The ultimate goal of the project is therefore the assessment of HDACs as potential drug targets for treating allergic airway inflammation. This project is embedded in research area 1 and research area 3. Collaborations for P04 within SFB: P02, P03, P05, P06, P08, P09.
Information about the project leader & team
P05 – Wilfried ELLMEIER: HDAC function in T cells beyond histone modifications.
We previously demonstrated that HDAC1-cKO mice are completely resistant to EAE, raising the exciting possibility of using selective HDAC1 inhibition to treat inflammatory and autoimmune diseases. Class I HDACs have non-catalytic scaffolding functions, and it is unknown whether HDAC1 regulates T cells through such scaffolding functions and/or through its catalytic activity. We will test the hypothesis that the inhibition of HDAC1 catalytic activity is indeed sufficient to induce EAE resistance. Furthermore, we will investigate the acetyl-proteome of primary activated CD4+ T cells and its cell type specificity in Th lineages. We hypothesize that Th subset-specific acetylation patterns on non-histone target factors regulate Th effector function and that HDAC-dependent changes in the activity of key non-histone targets contribute to EAE resistance in HDAC1-cKO mice.This project part is embedded in research area 1 and 2. Collaborations of P05 within SFB: P02, P07, P08, P09.
Information about the project leader & team
P06 – Iris GRATZ: HDAC function in peripheral regulatory T cell biology.
We have previously developed a mouse model that is uniquely suited to investigate in vivo T cell differentiation and skin immune regulation by skin-tropic Treg cells. Based on our preliminary findings, we hypothesize that HDACs control peripheral Treg (pTreg) generation in vivo, which raises the possibility that specific inhibitors might be employed clinically to regulate pTreg generation in response to skin-antigens (e.g. in skin autoimmunity or skin cancer). Class I HDACs are hypothesized to regulate Treg biology at several levels. In P06, we will test whether HDAC1 and HDAC2 (1) regulate pTreg generation in skin immunity, (2) impact Treg migration to the target tissue, and (3) we will assess pTreg function and lineage stability under inflammatory conditions. In summary, we will determine how HDAC-1 and HDAC-2 regulate chromosome accessibility and stability of Treg in vitro as well as in vivo using state of the art methods of Immunology and Molecular Biology.This project is embedded in research area 1. Collaborations of P06 within SFB: P02, P03, P04, P05, P08.
Information about the project leader & team
P07 – Markus HARTL: HDAC-dependent interactomes and acetylomes in T cells.
Although it is evident that HDACs are involved in T cell differentiation and the control of T cell mediated immunity, the mechanistic basis of this regulation is rather unclear. We have previously developed and optimized protocols for quantitative mapping of lysine acetylation on a proteome-wide scale. We are convinced that the application of this methodology to T cells is imperative to study how HDACs affect T cell function and development. We will test this by mapping the proteome and acetylome of Th subsets after HDACi treatment or in HDAC-cKO to determine HDAC-specific substrates and their dynamics. Moreover, we will map the interactome and post-translational modification dynamics of HDAC1, HDAC2 and selected substrates, in order to dissect catalytic and non-catalytic functions of the deacetylases and to evaluate the functional importance of their PTMs. P07 will provide an essential knowledge base for all members of the consortium, yield evidence for underlying mechanisms of HDAC function, and deliver a toolbox to test predictions on the proteome level.This project is embedded in research area 2. Collaborations of P07 within SFB: P02, P05, P08, P09.
Information about the project leader & team
P08 – Karl KUCHLER: HDACs and T cell-mediated antifungal immunity.
This project will exploit time-resolved in vivo/ex vivo RNA-seq and ATAC-seq focusing on the Th17 subset to identify class I HDAC-dependent GRNs controling Th polarization during fungal infections, including the discovery of evolutionary conserved HDAC-dependent GRNs in mouse and humans. Moreover, MS-based and acetylomics of mouse Th17 will define the genome-wide set of HDAC1 histone and non-histone targets, including changes upon fungal infections and after HDACi treatment. This will disclose infection-specific targets in Th17 subsets and uncover related GRNs relevant for skin infections, inflammation-driven autoimmunity, and allergic airway inflammation. Finally, prioritized candidate HDAC1 targets will enter a validation pipeline entailing gene dosage (GoF/LoF) in Th subsets and adoptive transfer into mice to verify beneficial/detrimental phenotypes on fungal clearance in vivo. This work will reveal, for the first time, a comprehensive picture how class I HDACs exploit cellular and molecular regulatory mechanisms to control T cell-mediated immunity to human fungal pathogens.This project part is embedded in all research areas. Collaborations of P08 within SFB: P02, P03, P04, P05, P07, P09.
Information about the project leader & team
P09 – Christian SEISER: Regulation of HDAC complex function in T cells.
We have recently demonstrated that catalytically inactive isoforms of HDAC1 and HDAC2 have dominant-negative effects on the activity of HDAC1/HDAC2 co-repressor complexes. We hypothesize that the expression of catalytically inactive HDAC1 and HDAC2 mimics the effect of isoform HDAC inhibitors. In P09, we will test this hypothesis by examining HDAC1/HDAC2 co-repressor complex activity, chromatin accessibility, gene expression and homeostasis in CD4+ T cells expressing inactive HDAC1 and/or HDAC2. Further, the catalytic activity of HDAC1 was shown to be modulated by lysine acetylation and sumoylation. We will analyze the effect of these posttranslational modifications on the regulatory function of HDAC1 in CD4+ T cells using acetylated HDAC1 as paradigm for a non-histone target of HATs and HDACs.This project part is embedded in research area 1. Collaborations of P09 within SFB: P02, P04, P05, P07 and P08.
Information about the project leader & team
Training
Postdocs and PhD students will work on cutting-edge research projects in a collaborative SFB research environment. All members of the SFB laboratories will actively contribute to the integration of new members employed by the SFB, ensuring excellent supervision and support. SFB PhD students will enroll in PhD programs at the hosting institutions. SFB principal investigators are faculty members of various thematic PhD programs at the MedUni Wien (including the MedUni Wien PhD program “Immunology”, the FWF-funded doctoral programs “Inflammation and Immunity” and “Tissue-Homeostasis in Health and Disease”) or at the University of Salzburg (FWF funded doctoral program “Immunity in cancer and allergy”).
SFB postdocs will be also fully integrated in the SFB research teams. In addition to research training, we will foster their career towards independent PI level, for example through participation in postdoctoral training networks at MedUni Wien (organized by the Immunology Research Cluster) or at CeMM, which will provide several opportunities for networking within and beyond Vienna and Austria.
We encourage students and postdocs to engage in SFB activities beyond scientific research. PhD students and postdocs will have the opportunity to meet invited seminar speakers and guest professors, to organize SFB annual symposia, and to participate in SFB dissemination activities to the public. Additional training will entail manuscript and grant reviewing whenever possible. Together, these activities will help our young researchers to establish their own personal networks and to acquire complementary skills, which will be helpful for their future career.
MedUni Wien PhD program "Immunology"
N094 PhD program "Immunology"
