A. Kainz (1)*, M. Kammer (1,2)*, R. Reindl-Schwaighofer (1), S. Strohmaier (3), V. Petr (4), O. Viklicky (4), D. Abramowicz (5), M. Naik (6,7), G. Mayer (8, 9), R. Oberbauer (1)
1- Department of Nephrology, Medical University of Vienna, Vienna, Austria
2- Institute of Clinical Biometrics, Medical University of Vienna, Vienna, Austria
3- Department of Epidemiology, Medical University of Vienna, Vienna, Austria
4- Department of Nephrology, Transplant Center, Institute for Clinical and Experimental Medicine, Prague, Czech Republic
5- Department of Nephrology, Antwerp University Hospital, Antwerp, Belgium
6- Department of Nephrology and Medical Intensive Care, Charité Universitätsmedizin Berlin, Germany
7 Berlin Institute of Health, Berlin, Germany
8- Department of Internal Medicine IV – Nephrology and Hypertension, Medical University of Innsbruck, Innsbruck, Austria
9- Austrian Dialysis and Transplant Registry, Innsbruck, Austria
* contributing equally

Cohort characteristics (Number of individuals in the analysis: 2346)
View PDF
Flowchart of patient inclusion and exclusion from the Austrian Dialysis and Transplant Registry, as well as outcome data.
View PDF
Hazard ratio comparing the effect of the treatment strategies ‘retransplant’ (TP) vs ‘never retransplant – remain on the waitlist with continued dialysis’ (no TP) on mortality, conditional on the waiting time in years elapsed since first graft loss. The hazard ratio was estimated using Cox proportional-hazards models incorporating stanilized inverse probability of treatment and stabilized inverse probability of censoring weights. The solid line shows the estimate using a single model fit on the full target trial dataset, grey bands indicate 95% confidence intervals obtained by 1000 bootstrap resamples. The dashed line presents a hazard ratio of unity (no difference) for reference.
View PDF
Treatment comparison in terms of survival outcomes. Upper panel (a) depicts the restricted mean survival time (RMST) difference estimated from the weighted Cox proportional-hazards model also shown in Figure 2. Each sub-panel gives the RMST of the transplantion group minus the RMST for the control group up to 15 years of follow-up (x-axis), conditional on the waiting time in years elapsed since first graft loss (GL, sub-panel header). Lower panel (b) depicts the corresponding estimated adjusted survival curves for both groups in an analogous manner. The difference in RMST in panel (a) is given by the difference in the area under both survival curves up until a specified time of follow-up, and is an estimate of the average difference in life-years when the two populations are followed for a certain amount of time. In all graphics the point estimates were obtained using a single model fit on the full target trial dataset, the shaded areas indicate 95% confidence intervals obtained by 1000 bootstrap resamples.
View PDF
Patient survival and death censored graft survival. Kaplan-Meier plots for (a) patient survival and (b) death censored graft survival after retransplantation, stratified by waiting time after first graft loss. Data is only shown for up-to 15 years of follow-up after retransplantation. This graphic was based on the 1869 individuals who had a second transplantation during follow-up.
View PDF
Summary of results for effect of second transplantation for all sensitivity analyses. CI denotes confidence interval
View PDF
Visualisation of study design which defines two main time axes. First, the time axis ‘time since first graft loss’: for each individual the graft loss after first transplantation (1. TP) defines the ‘baseline’, or time 0 (t = 0) of these individual time scales. Second, the time axes defined in each auxiliary trial: auxiliary trials are started at each observed transplantation (TP), representing time 0 for that particular trial, and the time to the outcome is measured from the start of the trial. All auxiliary trials are ordered by the first time axis, i.e. time since graft loss of an individual after the first transplantation. Only individuals who have joined the waiting list (WL), and did not receive a transplantation at that point in their personal history of follow-up since graft loss are eligible to join the auxiliary trial initiated by a TP. Thus, in the graphic above, in the first auxiliary trial starting with the 2. TP of individual 1, individuals 1, 3 and 4 would be eligible, in the second trial started by individual 2, individuals 2, 3 and 4 would be eligible, and in the third trial started by individual 3, individuals 3, 4 and 5 would be eligible. Study outcomes are indicated as filled (death) or empty (survived until date of data lock, loss to follow-up) diamonds. In the first trial, for individuals 1, 3, and 4 the time would be measured from the time of 2.TP of individual 1, to the respective individual outcomes. The outcomes in the auxiliary trials are modified to conform to the definition of the treatment strategies by censoring all individuals who received a transplant after joining an emulated trial at the time of their transplant.
View PDF
Sample size for each auxiliary trial. Each individual may be counted multiple times in this illustration. The black line gives to the total number of individuals entering a single emulated trial. The blue line gives the number of individuals who died during the follow-up of that trial (administratively censored at 15 years after trial start), while the red line gives the number of individuals who started treatment (received a transplant) during follow-up of that trial.
View PDF
Covariate distributions in each auxiliary trial, shown over time since first graft loss. The black lines give relative frequencies for binary variables and median values for continuous ones. The grey area indicates the interquartile range (25th to 75th percentile) of the distribution. ‘GL’ stands for graft loss, ‘TP’ for transplantation. Note that these distributions do not necessarily agree with the cohort characteristics reported in Table 1 of the main manuscript; the latter summarises the whole cohort entering the analysis at first graft loss, while this graphic shows the observed distributions after applying inclusion and exclusion criteria (in particular active waitlist status) in each auxiliary trial at specific time points after first graft loss. This represents the changing population characteristics conditioned on in the analysis over time. Apart from slight discrepancies in the first few months after graft loss (partly also attributable to small sample sizes in these auxiliary trials), most cohort characteristics stay comparable (also to Table 1) over the study period. Exceptions include age at trial start (which increases with time, as to be expected from the definition), time between first graft loss and waitlisting for second transplantation (which increases with time, as to be expected from the definition), and receipt of a living donor organ (the longer the waiting time the less likely it is to receive a living donor organ)
View PDF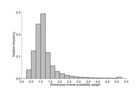
Histogram of relative frequencies of inverse probability weights used as observations weights in the final Cox analysis models. These weights were computed as the product of stabilized inverse probability of treatment weights trial to address confounding, and yearly stabilized inverse probability of censoring weights to address adherence to the treatment strategies of interest. The final weights were winsorized at their 0.5% and 99.5% percentiles to remove outliers with highly impactful weights.
View PDF
