Background
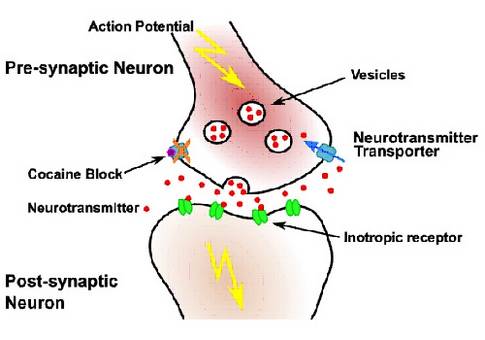
Neurons use electrical signals called action potentials to propagate information along axons and dendrites. Otto Loewi received the Nobel Prize in Medicine (1936) for the discovery that small molecules (neurotransmitters) are the information carriers in inter-neuron communication at synapses.
Neuronal signalling requires fast (milliseconds) removal of the released neurotransmitters. Several diseases are associated with improper neurotransmitter clearance. Recreational drugs like cocaine or ecstasy interfere with transport processes and lead to hallucinations, euphoric stimulation, addiction and extensive physiological and psychological problems.
NeuroTrans will focus on key NSS family members including the prokaryotic small amino acid transporter (LeuT), the dopamine transporter (DAT), and the GABA transporters (GAT1-3, BGT1). DAT is most prominently associated with reward and Parkinson’s disease, while GABA transporter deficiencies lead to epilepsy and seizures. Between 50 and 200 Novel Psychoactive Substances (NPS) appear each year on the illicit drug market. NPS are untested, neither for pharmacodynamic activity nor for toxicological effects, creating a huge burden for society. Thorough and rapid characterization of NPS is therefore a top priority, both for healthcare professionals and policy makers.
Overview of the Research Programme
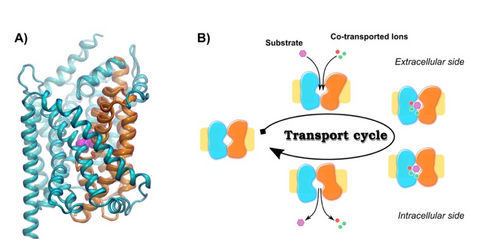
The key goal of NeuroTrans is to establish a framework for understanding Neurotransmitter:Sodium Symporters (NSS) that creates a direct link between the fundamental structural, biochemical and biophysical principles at the molecular level and the mode(s) of action of novel psychoactive substances, but also also of disease causing mutations.
To achieve this ambitious goal, NeuroTrans has defined the following work-packages:
-
WP1: Determining transporter structures and gaining insight into complex formation
To characterize ligand bound complexes and derive structural and mechanistic insights by determining GABA transporter structures. -
WP2: Characterizing electrophysiological ligand binding and substrate transport
To characterize the electrogenic events associated with transport (binding, translocation, release). Novel technologies for electrophysiological recordings will be developed by Nanion to significantly improve instrument sensitivity and throughput in vesicular and cellular recordings. -
WP3: Dynamic, thermodynamic and mechanistic insights into substrate binding and transport
To obtain dynamic, kinetic, thermodynamic, structural, and mechanics insight into the processes of substrate binding and transport with improved temporal and spatial resolution. NanoTemper will develop novel technologies for efficient measurements of thermodynamic and kinetics parameters in vesicles and cells. -
WP4: Mode of action of Novel Psychoactive Substances (NPS)
To identify the mode of action of Novel Psychoactive Substances (NPS). The development of new microfluidic devices by Elvesys for cell based measurements and the integration with the novel technology by Nanion and NanoTemper will boost throughput by orders of magnitude and significantly decrease the time-lag between initial discovery and complete characterization to provide policy makers and regulatory bodies with information on a useful time-scale. -
WP5: Molecular determinants of novel disease causing mutations
To assess the molecular determinants of novel disease causing mutations associated with neuropsychiatric disorders discovered in large population screens. We will dissect and model how discrete molecular perturbations caused by such mutations mechanistically elicit discrete changes in transporter function that in turn can translate into disease-relevant dysregulation of neurotransmitter homeostasis.
Methods
Fundamentally new insights are often only possible with the establishment of new experimental techniques or the integration of multiple methodologies. We will achieve this through instrument and method development to obtain higher resolution, both temporal and spatial, dynamic data and by integration of methods to obtain multidimensional readouts. ESRs will be educated according to the unified training objectives in the unique set of skills available within the NeuroTrans network. The most significant of these skills are highlighted here:
- Spectroscopy: Förster resonance energy transfer (FRET) spectroscopy, most importantly in single molecule FRET, transition metal FRET and multiparameter FRET with high time resolution (ns-μs), advanced time-domain EPR methodologies and NMR spectroscopy.
- Proton-deuterium exchange Mass Spectrometry (HDX-MS): HDX-MS, specifically applied to investigate 1H-2H exchange experiments of protein backbone amide protons.
- Structural biology (TRX, cryo-EM): X-ray crystallography (MX), electron microscopy (EM), and time-resolved X-Ray crystallography (TRX) at larger scale facilities such as Diamond (UK), ESRF (France) and PETRA III (Germany) synchrotron sources.
- Molecular biology: Molecular and cell biology for cloning, site directed mutagenesis, protein expression, cell culture and insertion of unnatural amino acids.
- Protein purification: Protein purification, reconstitution in proteoliposomes or nanodiscs and the assertion of maintained protein integrity and function.
- Biochemistry and Pharmacology: several biochemical techniques including cell and proteoliposome based transport assay, inhibition of transport, ligand binding assays, isothermal titration calorimetry (ITC), and substrate quantification to determine the biochemical and pharmacological kinetic and thermodynamic parameters.
- Electrophysiology: patch clamp technique in eukaryotes and oocytes to investigate the currents that are associated with the electrogenic substrate transport by the transporters.
- Molecular dynamics simulations: molecular dynamics simulations and advanced simulation techniques.
- Kinetic Modelling: development of kinetic models of the transport cycle based on coupled differential equations.