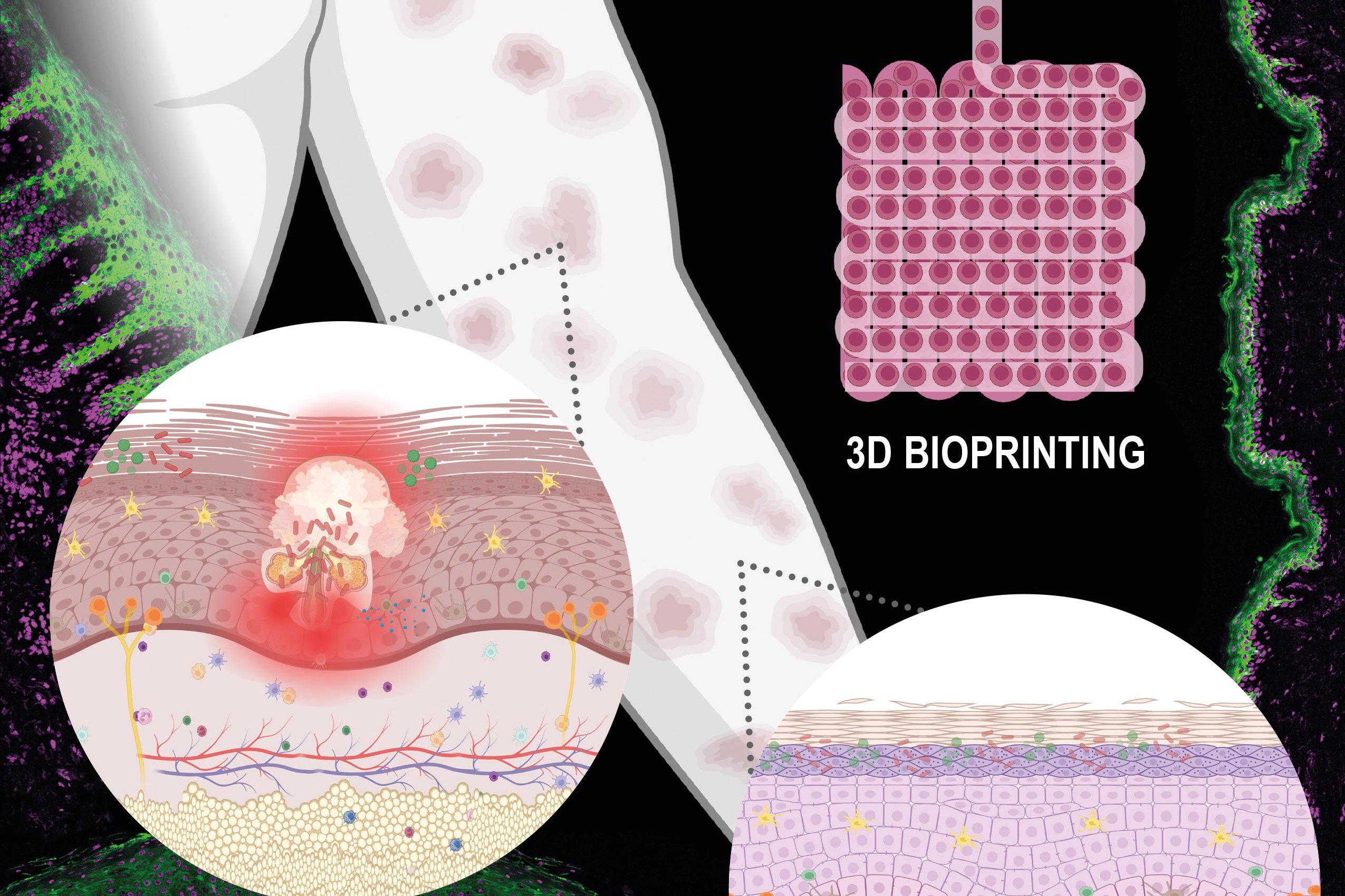
(Vienna, 17 December 2025) In cooperation with MedUni Vienna, researchers at TU Vienna have investigated how 3D bioprinting can be used specifically to produce complex skin models as part of a recent review. The study shows that, by using suitable biomaterials and precise printing technologies, it is possible to create multi-layered skin structures into which immune cells can also be integrated – a decisive step in advancing research into chronic inflammatory skin diseases such as psoriasis. The research paper has been published in the journal "Advanced Healthcare Materials".
Roughly one quarter of Europe’s population suffers from chronic inflammatory skin diseases such as psoriasis, eczema, or acne. Developing new therapies for these conditions is often difficult. Animal experiments – aside from their ethical concerns – frequently fail to produce reliable results, because animal skin differs greatly from human skin in both its anatomy and immune response. The so-called in vitro models are therefore needed to study skin diseases in laboratory conditions. A substantial challenge on the way to this goal – such models have to be immunocompetent, i.e. include all the necessary immune cells in order to mimic the situation in the real skin. The recent collaboration between the TU Wien and the Medical University of Vienna explored how the 3D bioprinting with biomaterials could fill this gap. This work is presented in a newly published review article in Advanced Healthcare Materials.
In Search of the Right in vitro Skin Model
"Different methods have been used in the past to create samples resembling human skin," says Georg Stary from the Department of Dermatology at the Medical University of Vienna.
"One option is to embed connective tissue cells into a collagen solution and culture them. However, this offers little control over the spatial structure, the resulting cell layer is not very stable, and it is difficult to integrate immune cells or blood vessels into the construct, which are instrumental for chronic inflammatory processes."
Another possibility is the so-called self-assembly method: connective tissue cells are cultured in the presence of large amounts of vitamin C, which stimulates them to build their own extracellular matrix that provides structural support. "But this process is very time-consuming and labor-intensive," says Stary. "And it lacks reproducibility – each sample develops differently, and we have little control over the structure that forms."
Skin from a Printer
"These are exactly the problems that 3D bioprinting can help to overcome, " explains study leader Aleksandr Ovsianikov, Head of the Research Group 3D Printing and Biofabrication, Institute of Materials Science and Technology, TU Wien. "The three-dimensional tissue is built layer by layer from living cells and carefully selected materials, in an automated fashion and in accordance to a computer aided design." Cells and hydrogels are combined into a viscous "bio-ink", which is then deposited in strands or tiny droplets – much like ink from a conventional inkjet printer.
At TU Wien it has been shown that the choice of hydrogel and cell types is crucial for the success of the model. Depending on the intended application, specially designed bio-inks are required.
Tailor-Made Structures for Different Purposes
Using 3D bioprinting technologies established at the TU Wien, skin models can be produced in a controlled and highly reproducible manner, allowing different diseases to be studied.
"We have developed psoriatic models containing T cells, the immune cells that trigger the chronic inflammation seen in psoriasis," says Andrea Gabriela Ulloa-Fernández (Research Group 3D Printing and Biofabrication, Institute of Materials Science and Technology, TU Wien). “With these models, we can investigate how the tissue responds to specific drugs."
Inflammatory models have also been produced using the 3D printing method to test anti-inflammatory substances. Even structures with blood vessels can be created – for instance, to study vascular damage in diabetes. "With our method, we can precisely define the architecture of the 3D model and the distribution of the extracellular matrix in which the cells attach and proliferate," says Ulloa-Fernández. "This gives us a completely new level of control over the final outcome compared to previous techniques. We hope that our artificial skin models will help move research on a wide range of skin diseases a significant step forward."
Publication: Advanced Healthcare Materials
Advances in Bioprinting to Model Immune-Mediated Skin Diseases.
Andrea Ulloa-Fernández, Marica Markovic, Julia Fernández-Pérez, Georg Stary, Aleksandr Ovsianikov.
DOI: 10.1002/adhm.202503806
https://advanced.onlinelibrary.wiley.com/doi/10.1002/adhm.202503806