(Vienna, 03 August 2017) APOSEC is a substance obtained from white blood corpuscles and was developed by a research group led by thoracic surgeon Hendrik Jan Ankersmit, Head of the Christian Doppler Laboratory for Cardiac and Thoracic Diagnosis and Regeneration at MedUni Vienna . Even during its preclinical development, it was demonstrated that the multifactorial agent can be used in heart attacks, strokes, spinal cord injuries and for healing wounds. This promising substance is now in the clinical phase of the approval process that will license it as a new drug for healing external wounds.
Before a newly developed drug can be used, it has to go through a laborious approval process – and the biologic substance APOSEC, consisting of soluble proteins, exosomes and lipids from white blood cells, developed and patented by Hendrik Jan Ankersmit and his team at MedUni Vienna, is no exception. The blood cells are first of all irradiated and release proteins during cell death, these proteins being known as a "secretome".
The "secretome" exhibits multifactorial therapeutic efficacy, as the research team was able to demonstrate, even during the early stages of preclinical testing. For example, it has an antibacterial effect, induces the regeneration of vessels and stimulates tissue healing. The cells themselves are no longer needed and are disposed of, since the therapeutic effect is the result of the combination of the various secreted constituents.
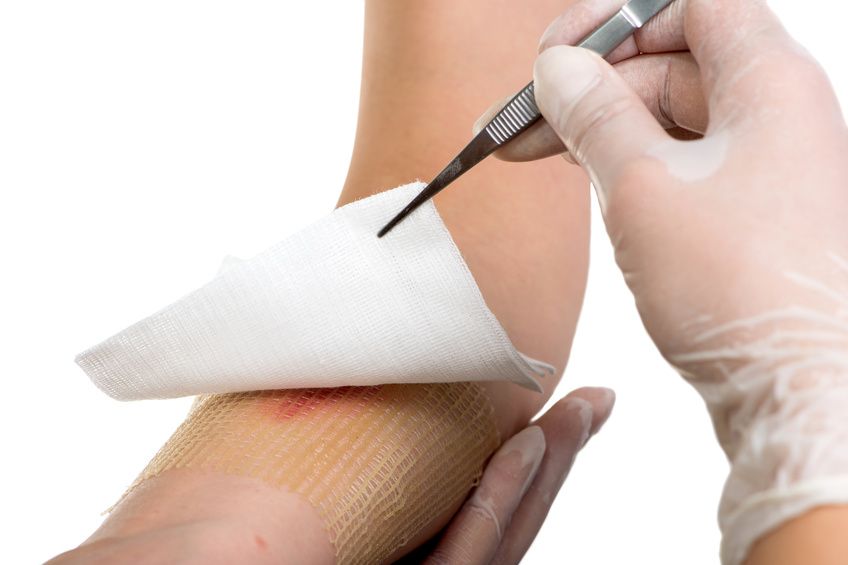
Human APOSEC, produced under GMP (Good Manufacturing Practice) conditions from the blood donation centre in Linz, which has been approved for clinical testing on humans by the authorities (Bundesamt für Sicherheit im Gesundheitswesen) is now being used in the clinical Phase 1 trial. The associated Marsyas-1 study is the first secretome-based dermal regeneration study in the world and has now been published in "Nature Scientific Reports". The study achieved its aim of demonstrating the safety of the active agent for dermal wounds. The requisite blood cells are obtained autologously, that is to say from the volunteers' own endogenous material.
The research team is now working on producing APOSEC from allogeneic cell material, that is to say material from other donors. This would allow cost-effective, large-scale production, so that the medication could be freeze-dried and kept available for immediate use. Based on the research findings so far, APOSEC promises to be suitable for a wide range of applications, for example for heart attacks, myocarditis, strokes and spinal cord injuries.
Successful joint project between several MedUni Vienna departments
The entire project was facilitated as part of a private-public partnership between MedUni Vienna, Christian Doppler Research Association (www.cdg.ac.at), the FFG (www.ffg.at) and Aposcience AG (www.aposcience.com). This "bench to bedside" development was and is a great success in terms of multidisciplinary collaboration between several divisions within MedUni Vienna (Division of Biomedical Research, Podesser; Division of Dermatology, Erwin Tschachler/ Michael Mildner; Division of Clinical Pharmacology, Michael Wolzt; Division of Cardiology, Mariann Gyöngyösi; the Clinical Trials Coordination Centre (KKS) and the entire Ankersmit working group).
Service: Scientific Reports
Safety and tolerability of topically administered autologous, apoptotic PBMC secretome (APOSEC) in dermal wounds: a randomized Phase 1 trial (MARSYAS I).
Elisabeth Simader, Denise Traxler, Mohammad Mahdi Kasiri, Helmut Hofbauer, Michael Wolzt, Christoph Glogner, Angela Storka, Michael Mildner, Ghazaleh Gouya, Alexandra Geusau, Carola Fuchs, Claudia Eder, Alexandra Graf, Michaela Schaden, Bahar Golabi, Marie-Bernadette Aretin, Susanne Suessner, Christian Gabriel, Walter Klepetko, Erwin Tschachler & Hendrik Jan Ankersmit. Scientific Reports 7, Article number: 6216 (2017)
doi:10.1038/s41598-017-06223-x