(Vienna, 17-07-2018) T-cells are central to immunity against pathogens and cancer. On their cell surface they express highly sensitive T-cell receptors, which allow them to detect minute amounts of pathogen- or tumor- derived antigens as a means to clear infections or kill abnormal cells. How T-cell receptors are distributed across the T-cell’s membrane has been the subject of intense research. New results obtained by a group of scientists from the Medical University of Vienna and the Technical University of Vienna now show, that current views have to be substantially revised.
Until now it has been thought that T-cells segregate their T-cell receptors within small membrane patches as a means to boost their sensitivity towards antigens. This idea is no longer supported by new data obtained by the Viennese team. Instead, a new concept is now emerging: T-cell receptors are organized on the plasma membrane in a randomized fashion to promote the fast and efficient detection of rare antigens. The study has large implications for the design of T-cell based therapies against cancer and autoimmune diseases and is now published in the journal „Nature Immunology“.
The needle in the haystack
“T-cells can be considered sentinels equipped with highly sensitive antigen detectors “, explains Gerhard Schütz, Professor and biophysicist at the Technical University of Vienna. “Every T-cell reacts for the most part to a distinct antigen. This is why we need in our body many different T-cells, each featuring a unique specificity towards a particular antigen “. Every T-cell expresses many thousands of copies of a T-cell receptor on its cell surface.
To fulfil their function as a sentinel T-cells are in constant communication with so-termed antigen-presenting or target cells. These display on their surface millions of different protein fragments - or antigens - via particular carrier proteins to be interpreted by the T-cells. The overwhelming majority of these protein fragments are derived from the body and are hence ignored by the T-cells. However, foreign antigens or those originating from mutated proteins are classified as a danger signal and illicit a strong T-cell response, which ultimately leads to the destruction of the source of danger, e.g. the pathogen, the virus-infected or cancer cell.
The T-cell’s search for the needle in the haystack begins with the formation of a transient contact with the antigen presenting cell. “You may look at it like this: among millions of different keys present on the antigen presenting cell, will there be a single one that fits one of the thousands of identical locks, the T-cell receptors, to ignite the T-cell’s engine?” explains Johannes Huppa, Professor and immunologist at the Medical University of Vienna.
What counts is efficiency
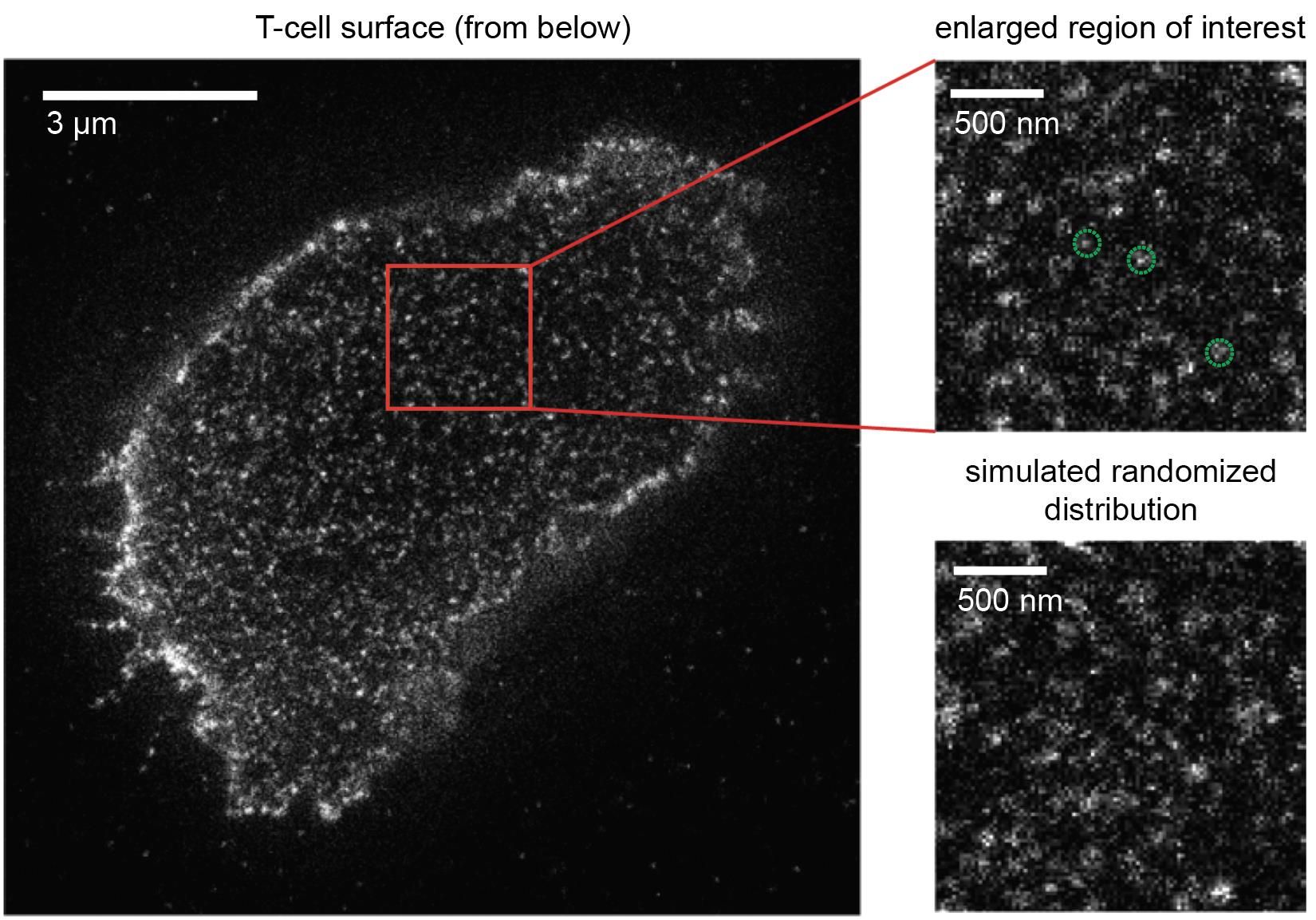
How T-cells manage to react towards foreign antigens in such a sensitive manner has been a matter of controversy for quite some time. A long-held theory has been that T-cell receptors are clustered as a means to engage antigens more efficiently. With the advancement of novel superresolution microscopy techniques, which allow for the visualization of individual T-cell receptors on living T-cells, this theory appeared to be validated at first: unusual structures were detected and interpreted as clustered T-cell receptors. But this conclusion was premature. “We first optimized microscopy methods and then took a very close look at the T-cells”, said Gerhard Schütz. It turned indeed out that such clusters were artefactual as they had resulted from imaging a single T-cell receptor multiple times.
The new Viennese study gives birth to a new concept: T-cell receptors are randomly distributed on the T-cell to accelerate rapid and efficient antigen detection for a vigorous immune response. No matter how the T-cell makes contact with the antigen presenting cell, there will always be an “ignition lock” in place for the right “key”, and “ignition” can swiftly proceed without delay. “These new insights are fully consistent with those of a recent study we published in Nature Immunology in May of this year”, comments Johannes Huppa. “We were able to demonstrate that T-cell receptors go it alone and do not hunt for antigen in packs, as was previously thought. A randomized distribution of these receptors, as we have now observed, makes a hell of a lot of sense when striving for maximum firepower to sensitize T-cells for antigen”.
Novel research tools for precision medicine of the future
“We are operating here at the ultimate limit of what can at current be achieved with most modern microscopy methods”, says Gerhard Schütz. Und Johannes Huppa adds: “These are very exciting days for immunologists like us. Molecular imaging catapults us into a position which enables us to visualize molecular processes in living cells in unprecedented detail. And comprehending the mechanics of life on a truly fundamental level is ultimately key to the successful development of patient-tailored immune therapies of the future, which we urgently need to combat cancer and autoimmune diseases such as type I diabetes or multiple sclerosis.
Service: Nature Immunology
TCRs are randomly distributed on the plasma membrane of resting antigen-experienced T cells
Benedikt Rossboth, Andreas M. Arnold, Haisen Ta, René Platzer, Florian Kellner, Johannes B. Huppa, Mario Brameshuber, Florian Baumgart und Gerhard J. Schütz; Nature Immunology, https://doi.org/10.1038/s41590-018-0162-7