University: University of Regensburg, Germany
PI: Christine Ziegler
ESR: Laure Gauthier-Manuel
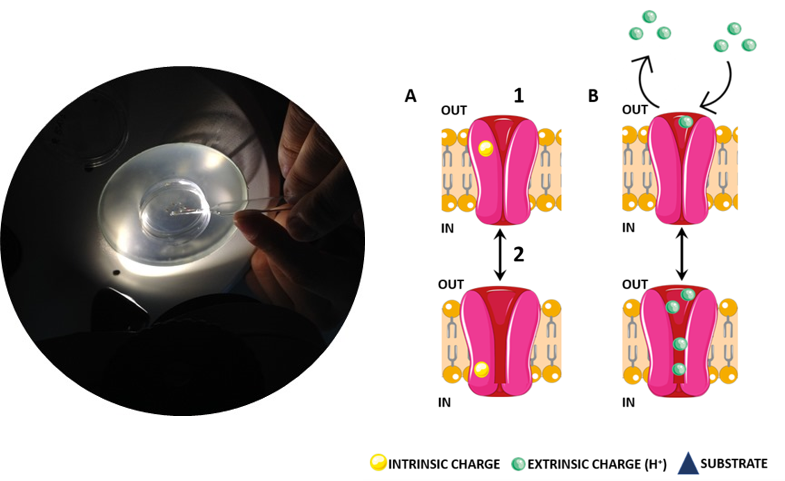
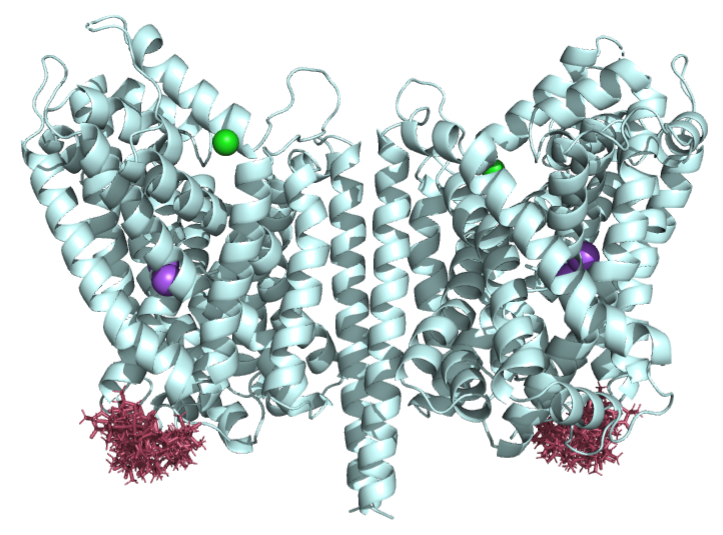
The project 1 concentrated on regulated transport of the osmolyte betaine in hyperosmotic stress response of renal cells. Medullar cells have to cope with extreme changes in extracellular osmolality from diuresis (low medullary solute concentrations) to antidiuresis (high medullary solute concentrations). The interstitial solution of the renal medulla is always fluctuant, and cells are constantly facing variable hypertonicity.
In general, cells counteract by changing the content of the remaining free cytoplasmic water through accumulation of ions and organic osmolytes facilitated by specific uptake systems, e.g. primary active ABC-transporters or secondary transporters. The equilibrium of both hydration and osmolyte concentration is an important physiological factor. Therefore, osmotically induced regulation of gene expression, metabolic activity and osmolyte transport is found in diverse tissues and organs, e.g. kidney, lung, cardiovascular system and musculoskeletal system, however the underlying mechanism and interplay of osmoregulated proteins is poorly understood.
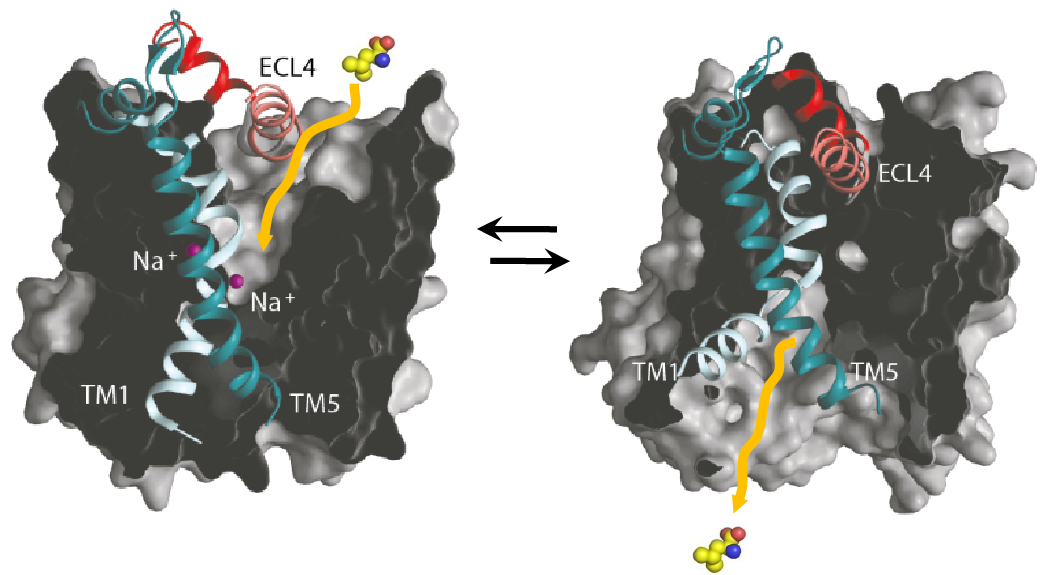
Transporters of the neurotransmitter-sodium-symporter (NSS) or solute carriers 6 (SLC6) family facilitate osmolyte transport in mammals. The first SLC6 subfamily includes transporters for the inhibitory neurotransmitter GABA (h/m/rGAT1, SLC6A1; mGAT4 = h/rGAT3, SLC6A11; mGAT2 = h/rBGT1, SLC6A12 and mGAT3 = h/rGAT2, SLC6A13) and for the osmolytes betaine (mGAT2/BGT1, SLC6A12), taurine (mTauT, SLC6A6) and creatine (rCRET, SLC6A8). Among those only BGT1 is strongly osmo-regulated both on transcriptional and on transport level.
We aimed for an investigation of the regulatory roles of lipids and post-translational modifications on the osmolyte and GABA transport in BGT1. To do so we want to determine the structure of BGT1 and BGT1-mimicking BetP mutants to address the following questions in molecular detail:
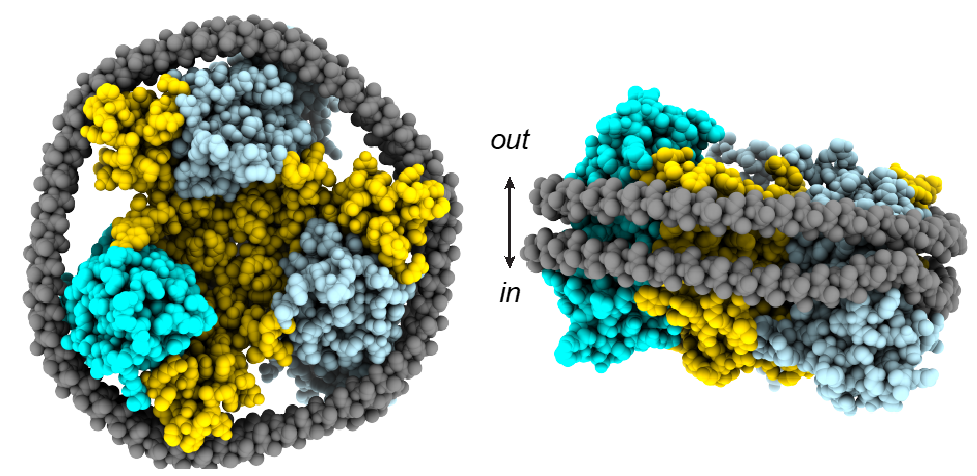
Identification of a GABA, betaine and ion binding sites in BGT1 – We will determine the atomic structure of BGT1 by X-ray crystallography and cryo-EM single particle analysis. In addition, the bacterial betaine transporter BetP will serve as a structural homologue for BGT1 to explain its characteristics in sodium coupled osmolyte transport. We will use the BGT1-mimicking BetP mutants to investigate GABA coordination further and based on our homology model could predict a third sodium-binding site in BGT1, which might facilitate high betaine accumulation by tighter ion coupling in BGT1.
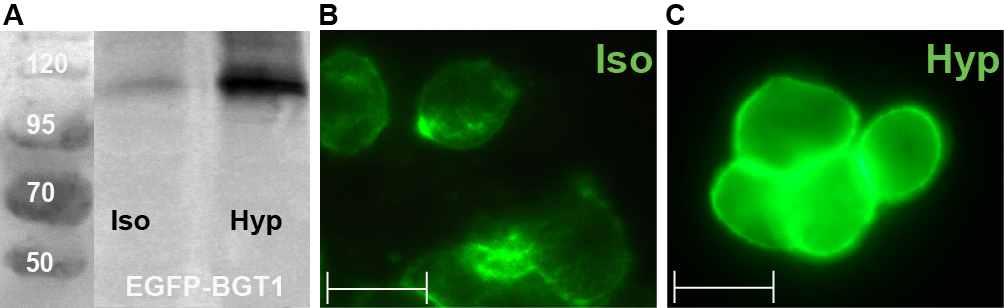
Regulatory role of BGT1 phosphorylation on serines and threonines - Protein kinase C (PKC) activation is known as regulator for many NSS transporters activating endocytosis-triggered depletion of transporters from the plasma membrane. To understand the influence of PKC phosphorylation on BGT1, several potential phosphorylation sites were mutated and tested with respect to their responses to PKC-mediated activators on the level of cellular distribution and transport properties. We will investigate these mutants structurally.
Regulatory role of N-glycosylation in BGT1 - N-glycosylation and its impact on the regulation mechanism of BGT1 was investigated in different cellular systems. We will continue studying the two predicted N-glycosylation sites (N171 and N183) in MDCK cells and oocytes and their individual role on BGT1 transport, regulation, stability and/or folding.
University: NOVA University of Lisbon, PT
PI: Eurico Cabrita
ESR: Alberto Daminato
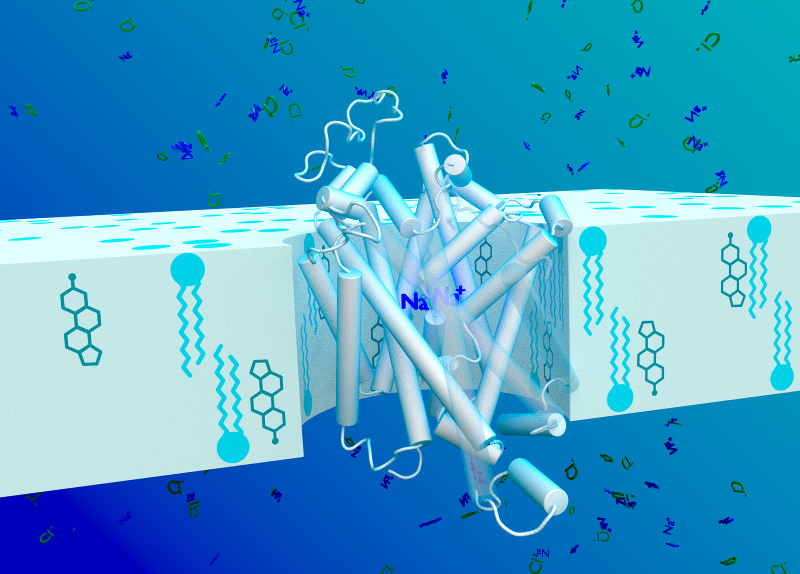
The core of this project is to contribute to the development of a comprehensive understanding of substrate binding and recognition through the experimental determination of ligand binding sites and poses. Membrane proteins are still a major challenge when considering NMR solution studies, mainly due to their large size and the need to use detergents encumbering the use of protein- observed solution NMR experiments.
In this project a variety of ligand and protein solution NMR experiments will be combined to investigate the transport cycle of LeuT and DAT, which the NeuroTrans consortium can purify in large quantities. Project 2 will be mainly focused on ligand-observed NMR experiments combined with protein-observed experiments using 13C LeuT methyl labelling (A and IVL) in order to establish structure/function relationships and derive information concerning the dynamics of the transport cycle.
The main objectives of project 2 are:
- to establish an NMR methodology to study binding/release of substrate and co-transported ions. This will be achieved in close collaboration with project 3, in order to develop protocols for sample preparation suitable for NMR studies, the use of proteoliposomes and encapsulated SMALPS will be explored.
- to determine binding poses of bound ligands based on ligand observation NMR experiments. STD-NMR and water LOGSY NMR experiments will be used in combination with different ligands (including inhibitors) in direct or competition experiments.
- to determine transporter dynamics during ligand binding, occlusion and release. This will be achieved in collaboration with project 9 and 12 in order to integrate magnetic resonance driven information with modelling data to study the conformational changes of the transport cycle.
University: University of Copenhagen, DK
PI: Claus Løland
ESR: Iris Elpida Kalenderoglou
The neurotransmitter transporters (NSSs) are membrane proteins located to the presynaptic terminal on neurons. They control neuronal communication by rapid reuptake of neurotransmitters. The rewarding effects of cocaine is due to its inhibition of the NNS for dopamine (DAT). There is no medical treatment for cocaine addiction. The atypical DAT inhibitors (ADIs) do also inhibit DAT, but they are not rewarding. Rather, they antagonize the effect of cocaine, making ADIs possible cocaine antidotes. The mechanism behind this is largely unknown, and the further exploration of this conundrum has been hampered by experimental limitations in the purification of functional DAT. We are the first to report the purification of functional DAT wild type.
The purpose of this project is to elucidate the fundamental differences in binding dynamics between cocaine and ADIs with advanced pharmacological technologies and state-of-the-art biophysical methods. This could include structural determination. We will find the molecular determinants that constitute the distinct pharmacological signatures of ADIs and cocaine. Our profound expertise in investigating protein conformational dynamics allows us to elucidate the specific DAT conformations induced by the two drug classes. Specifically, we will measure distances between specific DAT domains and how they are influenced by ligands. We wish to map the global structural dynamics induced by ligand binding using e.g. FRET-based methods and unnatural amino acids. Together, they could reveal a conformational ‘fingerprint’ for each drug class enabling a prediction for categorizing novel compounds.
We expect that these findings will provide novel information on complex conformational dynamics on a hitherto unpreceded level of detail and provide fundamental insight in why some DAT inhibitors are stimulants while others are not. This will contribute significantly to the development of a drug against cocaine abuse.
University: Medical University of Vienna, AT
PI: Thomas Stockner
ESR: Erika Lazzarin
No crystal or cyro-EM structures of a GABA transporter are available. Initially in this project, models of GABA transporters will be developed using the structures of the closely related serotonin transporters. Models, first evaluated using structure quality assessment methods, will be tested against modern force fields, which were shown to be able to detect also small structural deficiencies. Simulations will be carried out in close collaboration with Projects 1, 5, 6, 7, 8 and 11, which are experimentally studying the same transporters.
Project 4 will make extensive use of advanced simulation methods on HPC clusters to investigate the transport cycle of GABA transporters with the goal to obtain a comprehensive understanding of GABA transporter function. Within the NeuroTrans consortium, simulations will serve as a hub for data integration for deriving a holistic model of GABA transporter function. The main objectives of Project 4 are:
- to collaborate with Projects 1, 5, 6, 7, 8 and 11 through model creation and computational validation of preliminary data and to evaluate hypothesis derived from experimental data. Interaction will be especially intense with Project 1 for evaluation of preliminary X-Ray and cryo-EM structures.
- to directly study ion and substrate binding under varying transmembrane voltage conditions using the computational electrophysiology approach and compare to experimental data of Project 5 and 6. In addition, a direct identification of the ion binding sites should be possible.
- to use a set of advanced sampling methods to simulate the transport cycle, thereby investigating the steps of substrate and ion binding, substrate occlusion, transition to the inward facing state and cargo release. Data validation will be carried out by comparison to experimental data obtained in Projects 1, 5, 6 and 11.
- to quantify the free energy profile of substrate transport to identify the interactions triggering transport, using at least 3 different approaches: Metadynamics, Accelerated Weight Histogram method and Potential of Mean Force calculations.
Beneficiary: Nanion Technologies GmbH, DE
PI: Andre Bazzone
ESR: Rocco Zerlotti
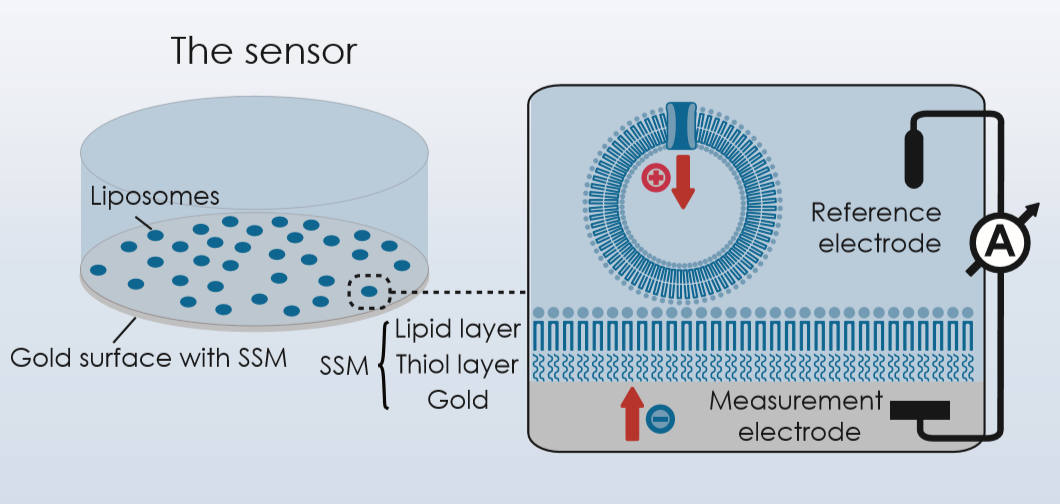
Our aim is the electrophysiological characterization of LeuT, DAT and GABA transporters using Solid Supported Membrane (SSM) -based electrophysiology. Our SURFE2R technology allows for real-time measurements of charge movements in the transmembrane electric field, originating from substrate translocation or electrogenic conformational transitions.
The three main objectives are:
- to develop an assay to enhance sensitivity and time resolution using SSM-based electrophysiology to increase signal-to-noise ratio for transport assays. This includes variations of the sensor surface and application of membrane potentials by chemical means.
- to electrophysiologically characterise NSS function using the SSM-based electrophysiology approach (EC50, IC50, Vmax, cooperativity).
- to develop an overall kinetic transport model by identifying and characterizing Na+, substrate and inhibitor induced electrogenic partial reactions of the transport cycle. Key is comparison of wild type transporters and transport deficient mutants as well as comparison the results obtained within NeuroTrans. Functional symmetry using different sample preparations also will be investigated.
There will be secondments with the research group of Christine Ziegler (University of Regensburg) and Thorben Cordes (University of Munich) which involve transporter assays using radioactivity labelled substrates as well as fluorescence techniques.
University: University of Insubria, IT
PI: Elena Bossi
ESR: Manan Bhatt
The aim of this project is to comprehend how sodium electrochemical gradient energizes the translocation of the substrates in the various steps of the cycle of GABA transporters and define in these states the role of chloride. The steps of the transport cycle of NSS proteins are associated with movements of charges (co-transported or intrinsic) through the membrane electric field. The sodium ions and substrates interaction in each step will be investigated by examining the currents (pre-steady state, leakage and steady-state currents) recorded in different conditions: altering driving force by varying intra- and extra-cellular concentrations of Na+ and Cl-, changing substrate and inhibitors, temperature, pH and voltages.
The biophysical data will be integrated with the uptake data: acquiring, in the same experiments, in a radiolabelled free approach, the amount of substrate entered the cell. These data allow to define electrogenicity and transport stoichiometry. This study will help to develop a complete model of the transport cycle in the presence of the substrate and of the inhibitors. Wild type, mutants, and chimeric GABA transporters will be analysed.
Mutants and chimeric protein will be prepared according to the prediction of the models of the project 4 and 9 or proposed by structural knowledge from project 1. Determinants considered essential in binding and moving ions and substrates across the membrane will be generated and investigated. Moreover, in this project, some other SLC6 proteins will be studied in parallel because NSSs share the transport mechanics while differ in co-transported ion stoichiometry and in chloride dependence. Data about mutants will be collected in collaboration with project 5 and support the project 13.
The main objectives of Project 6 are:
- determine the stoichiometry of the GABA transporters, developing a radiolabeled free method coupled to electrophysiological recording
- define the steps, rate and kinetic parameters of the transport cycles, investigating ion and substrate binding under vary conditions as described before and compare to experimental data of Project 4 and 5.
- Functional identification of the ion binding sites and of the residues involved in the main steps of the transport cycle, supporting the indications of Project 4.
- identify general valid principles through the comparison between the NSS transporters. Data validation will be carried out by comparison to data obtained in Projects 1, 4, 5, and 11.
University: University of Groningen, NL
PI: Dirk Slotboom
ESR: Zaid R. Anshari
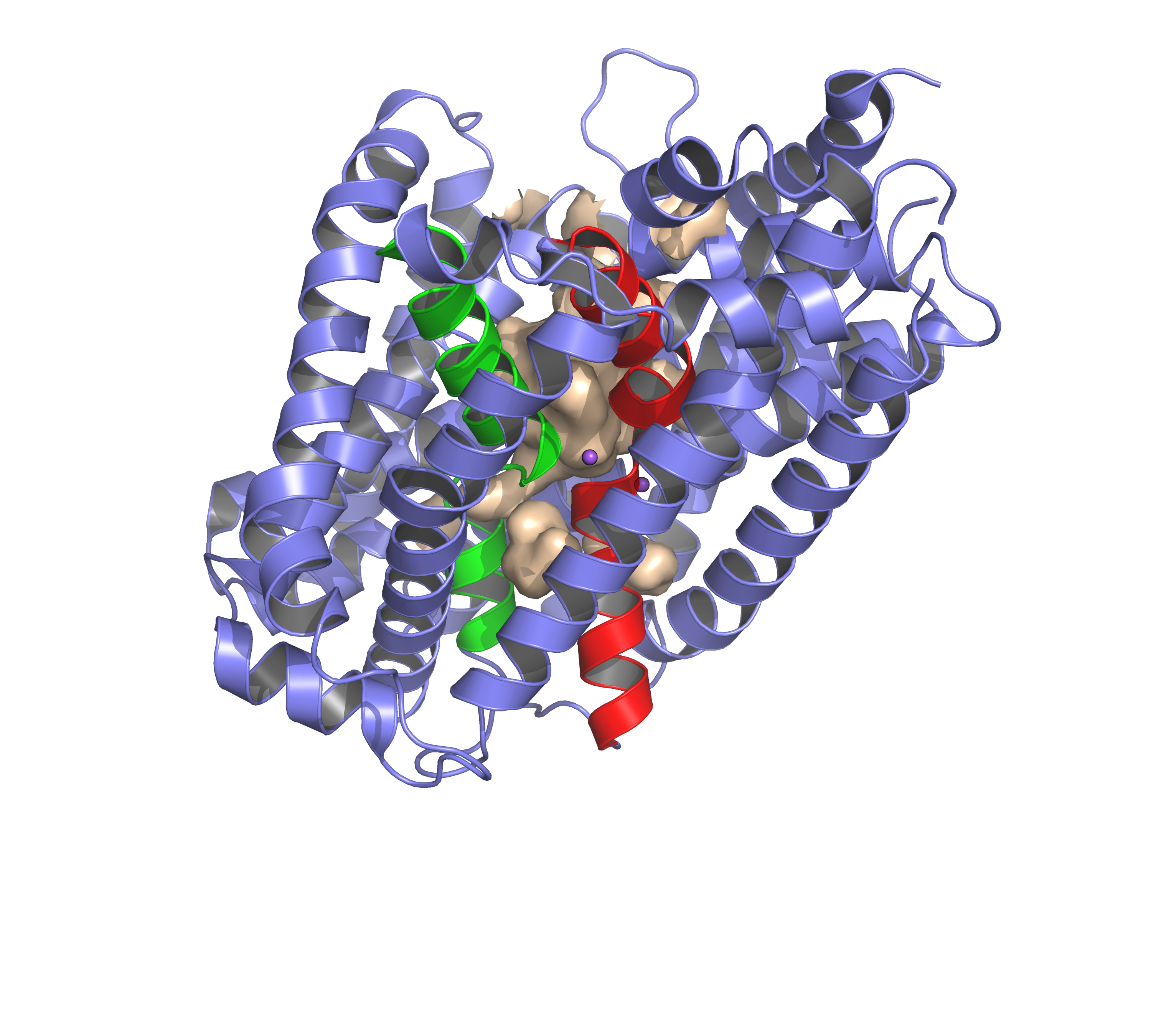
The available high-resolution structures of transporters from neurotransmitter transporters in multiple conformations allow for qualitative assessment of the principles of alternating access, but quantitative mechanistic understanding of coupling is far from complete. Knowledge of the kinetics of the transport reaction, which determines the order of binding of substrate and co-transported ion(s), and the sequence in which all relevant states are visited by the transporter, will help to reveal the coupling mechanism.
In this research project, we will lay out the kinetic schemes by combining mathematical models with data from classical enzymatic methods. Mutagenesis, single particle cryo-EM, time-resolved crystallography, single molecule FRET methods and EPR will be used to complement the enzymatic approach. In this project the PhD student will
- test potential kinetic schemes using transport and binding assays
- experimentally manipulate the energy-coupling mechanism using mutations
- solve structures of relevant conformational states of mutants with altered coupling properties
Beneficiary: NanoTemper Technologies GmbH, DE
PI: Nathan Adams
ESR: Anton Turaev
Science can only advance as much as enabling technologies allow for. NanoTemper is at the forefront of instrumentation development through its advanced technology that combines optical measurement technologies with the generation of microscopic temperature fields using IR-lasers. Using these microscopic temperature manipulations the goal is to measure local thermodynamic parameters of protein-ligand interactions. Within this project, novel experimental approaches will be combined with instrument and software development to measure protein-ligand interactions in more challenging and biologically relevant environments.
The main objectives of project 8 are:
- Determine transporter-ligand interaction parameters for ligands of GAT1, DAT and LeuT using currently available technologies.
- Develop novel approaches and instrumentation to measure transporter-ligand interactions (for in vitro or in vivo cell based assays).
- Develop software for measurement control, data acquisition and analysis.
University: Medical University of Vienna, AT
PI: Thomas Stockner
ESR: Leticia Alves da Silva
The core aim of this project is the development of a comprehensive model of the transport cycle of the human dopamine transporter (DAT) and of the bacterial small amino acid transporter LeuT. While LeuT has been crystallised in three conformations, no crystal or cyro-EM structures of the human DAT is available. The availability of structures of the closely related human serotonin transporter and the drosophila DAT allow for building reliable models.
First, DAT models will be evaluated using structure quality assessment methods. These will then be tested against modern force fields using extensive molecular dynamics (MD) simulations, as is was shown that simulation are able to detect also small structural deficiencies. Simulations will be carried out in close collaboration with the Projects of NeuroTrans, which are experimentally investigating on the same transporter. Access to HPC clusters allows for extensive use of advanced simulation techniques. DAT and LeuT will be studied in parallel, because sharing the transport mechanics. Comparison allows for separating transporter specific properties from general valid principles. The main objective of Project 9 are:
- to study the conformational changes of the transport cycle. Advanced simulation methods including Metadynamics, Accelerated Weight Histogram and the Potential of Mean Force calculations will be used to study transporter dynamics.
- to identify energy barriers, which separate states along the path of substrate transport.
- to characterize ligand binding in DAT, which in case of substrate (dopamine) leads to translocation, in presence of inhibitors (cocaine) leads to an arrest, while releasers (amphetamines) invert the mode of action and lead to substrate efflux.
- to elucidate the effect of disease causing mutation on DAT structure, dynamics and function, studied in silico through µs long unbiased simulations. Theoretical studies will be carried out in close collaboration with the Projects experimentally studying the same mutations.
University: Hamburg University, DE
PI: Arwen Pearson
ESR: Judith Estengre Pérez
Time resolved structural studies are the new frontier in structure biology. In a time-resolved experiment we aim to directly visualise the conformational changes associated with biological macromolecular function. To date, no time-resolved structural data for an NSS family member exist, although we do have structures of putative intermediates along the transport pathway.
Project 10 aims to directly visualise in real-time, with high spatial resolution, the structural changes that couple substrate binding to transport of at least one of the NSS family members using time-resolved crystallography.
This requires determining the optimal sample delivery method for each system and developing a reaction initiation protocol, mostly likely using a photocaged substrate. In this project we will explore the possibilities offered by both X-ray and electron crystallography. For the latter, the ability to carry out diffraction studies under ambient conditions is important, and so a key aspect of the project will also be the development of suitable sample environments for room temperature electron diffraction experiments.
This project will be carried out in close collaboration with the rest of the network. The highly pure NSS family members that are generated in the other projects will be targets for microcrystallization to yield the samples needed for this project. The data we obtain in this project will be fed into the molecular modelling, in combination with the spectroscopic and biochemical data obtained in the other projects, to allow us to build a detailed molecular description of how this class of membrane transporters work.
University: LMU Munich, DE
PI: Thorben Cordes
ESR: Despoina Kapiki
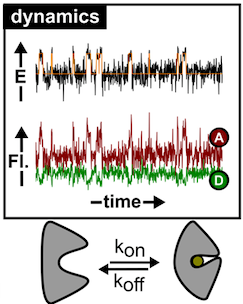
In this project, we will determine conformational states and distributions of GAT1 and its structural homologues to understand how structure is related to transport. The entire functional cycle will be studied with different single-molecule approaches on timescales ranging from microseconds of seconds. The methods include fluorescence microscopy, single-molecule detection schemes via confocal and TIRF microscopy, and multi-parameter fluorescence spectroscopy. We expect to achieve a detailed description of all essential steps within the transport cycle for different biochemical conditions and lipid compositions. In the project we will collaborate with Christine Ziegler (UREG) to develop protocols for transporter purification and reconstitution and we will work with Fraser MacMillan (UEA) to obtain complementary EPR measurements.
The main objectives are:
- to use single-molecule methods such as fluorescence quenching, anisotropy and FRET to resolve all relevant conformations of the GABA transporter GAT1 and that of structural homologues, using reconstituted proteins
- to study influence of biochemical conditions such as ion and substrate concentrations, inhibitors or functional mutations in the transporter
- to determine structures and conformational ensembles of unresolved, but regulatory important regions in GAT1 by combining smFRET with ab initio structure prediction methods in detergent environment, reconstituted GAT1 in nanodiscs- and proteoliposomes
- to study the effect of the signalling lipid PIP2 and of regulatory proteins on the conformational dynamics; this will be complemented by a systematic study on the effect of distinct lipid compositions on transport and regulation
- to carry out multi-parameter fluorescence studies and compare the results with others from the network to determine the set of exchanging conformers and establish their sequence in the transport cycle
University: University of East Anglia, UK
PI: Fraser MacMillan
ESR: Petros Tsalagradas
Accurate structural determination of different conformational states during the transport cycle remains challenging, which is further complicated by varying degrees of conformational distribution. ESR12 will employ advanced EPR spectroscopy to derive structural information in form of distances and angles constraints for state-specific structural ensembles. The use of multiple labels and especially the integration of both NMR and EPR techniques and their application to membrane proteins is a novel and innovative aspect of this project.
The specific objectives of ESR12 are:
- to establish an efficient spin labelling protocol for a set of cysteine variants to study conformations and dynamics of LeuT and DAT in classically reconstituted proteoliposomes and in detergent-free encapsulated SMALPS.
- to determine coarse-grained distance restraints of the transport cycle using on DEER/PELDOR and/or RIDME distance measurements.
- to establish in close collaboration with ESR2 a combined NMR and EPR approach to determine transporter dynamics during ligand binding, occlusion and release.
- to develop a multi-label triangulation approach using iDEER and STD-NMR to measure distances and orientations.
University: Medical University of Vienna, AT
PI: Harald Sitte
ESR: Ana Sofia Alberto e Silva
Between 50 and 100 Novel Psychoactive Substances (NPS) appear each year on the illicit drug market. Neurotransmitters transporters are located in presynaptic specializations. They inactivate neurotransmitter-mediated neurotransmission following exocytotic release by a simple reuptake mechanism. Recent crystallographic examination of mammalian neurotransmitter transporters provide a structural scaffold which supports transport by an alternative access mechanism. Monoamine transporters are a target of clinically relevant drugs: (i) antidepressants competitively block the reuptake of monoamines. Thereby, these compounds enhance the extracellular monoamine concentration which is relevant for clinical success. (ii) amphetamines and cathinones, which behave as substrates of the transporters, trigger non-exocytotic neurotransmitter release (efflux). The exact molecular mechanism of the psychostimulant action, however, still remains enigmatic. Novel psychoactive substances comprise psychostimulants, primarily the chemical substances classes of amphetamines and cathinones.
They are untested illicit compounds that carry perils, but also hold promises as future medication. Before moving from a possibly dangerous and toxic compound to a druggable lead, thorough characterization of its mode of action is essential. In parallel, ex- ploitation of in house available compound series of amphetamine and cathinone with specifically designed substitutes allows for enlight- ening structural and functional properties of DAT. The combination of the two aims will allow for gathering useful information for policy makers and indicate a potential for exploitation in the long-term future. In addition, our amphetamine and cathinone tool compounds will further elucidate the subtle differences between transported substrates and non-transported inhibitors. The objectives of ESR13 are: (i) to elucidate the mechanism of action of psychostimulant NPS by application of biochemical tracer flux assays, binding assays and elec- trophysiological approaches. (ii) to test already available amphetamine and cathinone compound series to identify changes in biochemical transport parameters. (iii) to specify the properties that discriminate substrates from an inhibitor.
Beneficiary: ELVESYS, FR
PI: Lisa Muiznieks
ESR: Francesca Romana Brugnoli
The core aim of this project is to establish a novel high-throughput system for the characterization of pharmacological substance using microfluidic technology. With inputs from Projects 6, 13 and 15, Project 14 will use extensive knowledge and existing flow control equipment of Elvesys to develop a microfluidic cell perfusion station allowing for flexible, fast, controlled and switchable sample application. Integration of in vitro cell experiments with temperature control and online detection will be achieved in collaboration with Projects 7, 13 and 15 so that the whole system will enable efficient assessment of DAT mutants using classical pharmacological approaches.
The main objectives of Project 14 are the following:
- to develop a microfluidic cell culture, multiple substrate testing and optical monitoring of cells under biocompatible conditions (temperature, compound delivery, perfusion station that allows flow control).
- to determine transport activity of DAT by optical detection of the fluorescent dopamine like substrate (ASP+).
- extension of the device to follow substrate transport in real time in GABA transporters (GAT1-3, BGT1) in collaboration with Project 1.
Beneficiary: University of Copenhagen, DK
PI: Ulrik Gether
ESR: Anna Campana
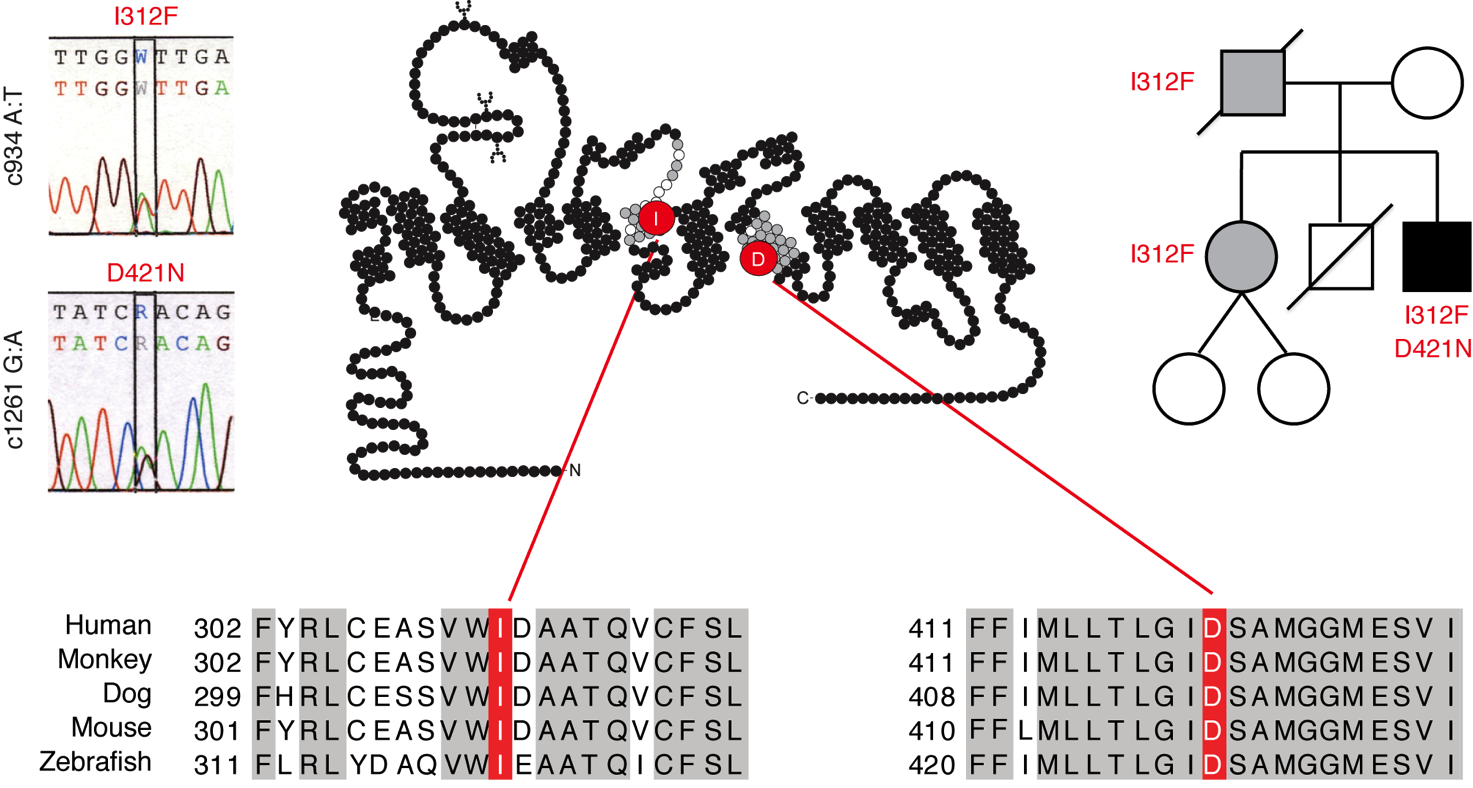
Recent data suggest that rare variants in the gene encoding DAT (DAT1) can play a hitherto unknown, but important role in the pathophysiology underlying both neuropsychiatric and neurodegenerative disorders. Despite that a number of mutations have been functionally characterized, it is still poorly understood how molecular perturbations in DAT are linked to distinct alterations in dopamine homeostasis in the brain of the patients. Interestingly, data suggest that the pathophysiological outcome might not only be a result of compromised DAT function but also involve “gain of disruptive functions” such as constitutive reverse transport of dopamine, enhanced ion permeability and ion channel-like functions.
The overall goal of this project is to map specific structural perturbations, elicited by disease-associated DAT mutations found in a large Danish cohort of 13,000 psychiatric patients, and link these perturbations to functional molecular phenotypes that in turn will be responsible for distinct alterations in dopamine homeostasis.
The specific objectives of Project 15 are:
- to assess the basic function of the mutants in high-throughput uptake and efflux assays as well as radioligand binding assays upon expression of mutants in heterologous cells
- to determine cellular distribution using live imaging and fluorescently tagged cocaine analogues
- to screen mutants for ion-channel-like properties using fluorescent voltage sensors and investigate the precise electrophysiological properties of selected mutants using two-electrode voltage clamp in Xenopus oocytes
- Make purified preparations of selected mutants (using the purifiable drosophila DAT as model system) and employ HDX-MS to assess mutation-elicited global conformational changes and FRET based techniques to map discrete intramolecular structural rearrangements
- Analyze data in context of molecular dynamics simulations and computational algorithms in order to build predictive models of how distinct types of molecular perturbations can lead to converging functional alterations.