This "Forschungsgruppe" is a highly interdisciplinary research team from the Institute of Cancer Research of the Medical University of Vienna together with the Institute of Analytical and Inorganic Chemistry from the University of Vienna.

Due to improved diagnosis and therapy, survival of cancer even at an advanced stage of the disease is increasing. Noteworthy, besides the novel forms of systemic cancer therapy, including targeted compounds and immunotherapy, also classical chemotherapy e.g. with platinum drugs is still an important and effective therapeutic strategy.
Supported by the novel high-end technologies and the massive increase of know-how concerning the nature of cancer, also chemotherapy gets more precise and effective. Additionally, it becomes increasingly obvious that also for metal-based chemotherapy precise targeting mechanisms exist. Thus, not only the direct cytotoxic activity against the tumor cells, but also e.g. immune effects are important for a successful therapy response. However, still positive outcomes are not achieved in every patient and side effects, although nowadays much better manageable, are still one of the key limiting factors of cancer cure.
Consequently, our cooperative project network aims to improve the quality of platinum-based systemic cancer therapy in several directions:
- We want to analyze the fate and the impact of clinically used and experimental platinum compounds down to the single cell level of the cancer tissue comprising in addition to the tumor cells also blood vessels, stroma and immune components. For these single cell analyses, high-end analytical techniques are available in our consortium and will be optimized in course of this project.
- Concerning novel remedies, the focus will lie on manipulating not only the cancer cells but also the direct environment of the tumor (as the surrounding tissues like stromal cells, immune cells etc. is often “abused” and altered by the tumor to support its needs). This should allow improved tumor control and reduced therapy failure by resistance development often caused by alterations in both tumor cells and microenvironment. Moreover, besides enhancing the anticancer effect, we aim to reduce the side effects by activating the cytotoxic activity of the metal drugs specifically inside the malignant tissue.
From old to new: Platinum drugs heading for the future
Strategically, already successfully used platinum drugs will be altered (oxidized) in a way that leads to reduced toxicity in the health tissue, and the ability to synthetically attach up to two additional chemical moieties (including other drugs supporting the platinum compound or delivery structures allowing to enhance accumulation in the malignant tissue). Consequently, only in the chemically altered environment of the malignant tissue, these per se non-toxic compounds (prodrugs) should be activated and release their cell killing activities.
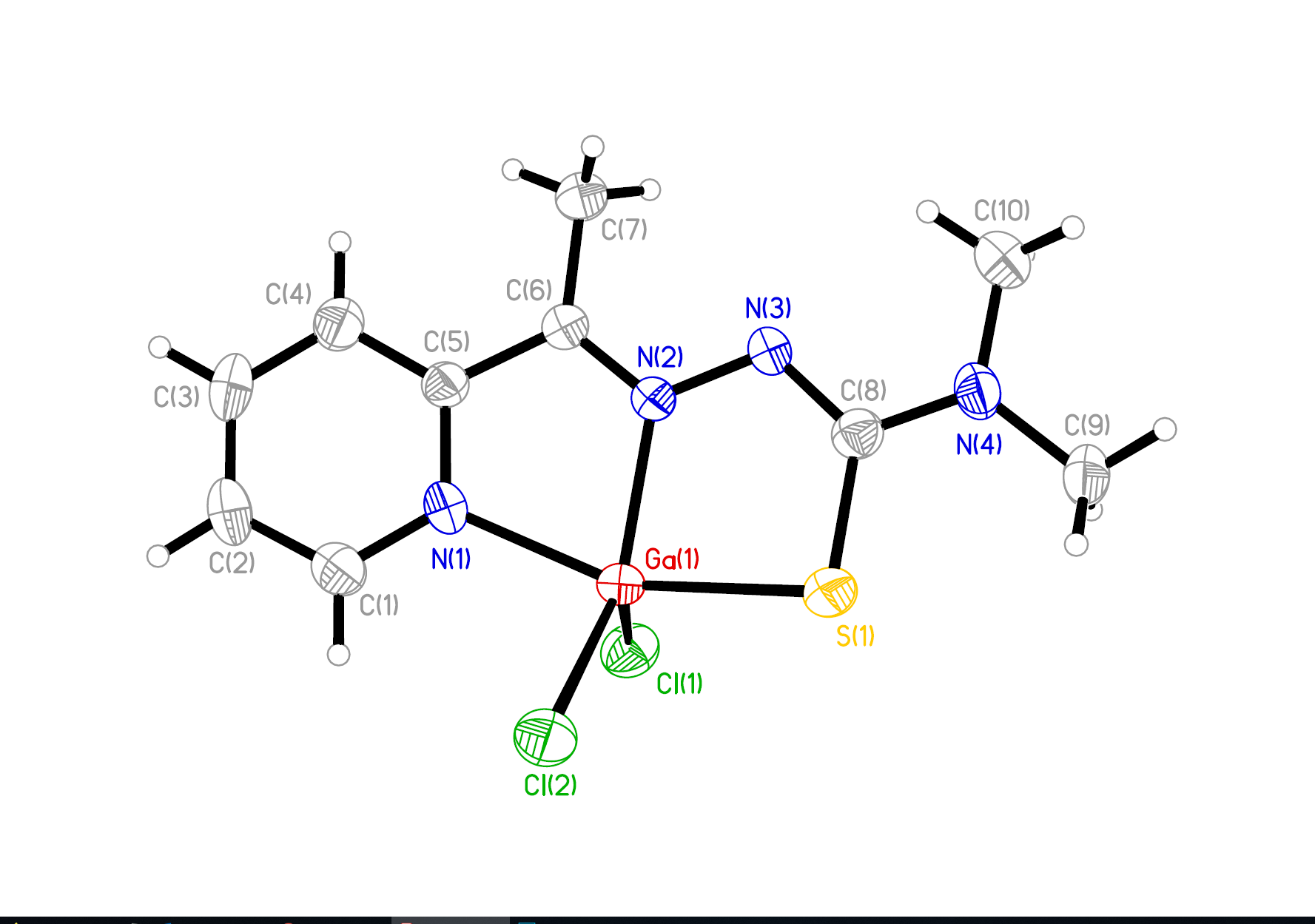
In order to fulfill such a challenging task successfully, multidisciplinary cooperations like in this consortium are essential, including synthetic chemists (generating the novel remedies), analytical chemists (able to precisely determine the fate of the remedies on their impact on the organism), as well as cancer biologists and toxicologists (to test the compounds´ activities and adverse effects using diverse models systems). In detail, our strategies comprise the development of anticancer platinum prodrugs specifically targeted to the tumor (via binding to the main blood protein albumin which is efficiently degraded by the tumor but much less by health cells) and releasing in the malignant tissue besides the cytotoxic chemotherapy (cisplatin, oxaliplatin) also immune-stimulating agents. Finally, therapy response/failure will be traced back to the contribution of metabolic factors and the tumor architecture down to the single cell level with a specific focus on the role of fibroblasts and immune cells.
Research Group

Univ.-Prof. Mag. Dr. Walter Berger
Center for Cancer Research
Department of Medicine I
Medical University of Vienna
Borschkegasse 8a
1090 Vienna, Austria
T: +43 (0)1 40160-57553
F: +43 (0)1 40160-957502
Research interests
The research group focusses on a translation of basic research data towards clinical application to foster the understanding of cancer therapy responses and the causes of intrinsic and acquired therapy resistance. Moreover, we develop systemic cancer therapy with a focus on notoriously therapy resistant cancers including primary brain tumors, thoracic malignancies and sarcomas.
- Identification of novel therapeutic molecular targets and the respective inhibitors
- Development of tumor-targeted small molecule anticancer agents and smart metal drugs with tumor-specific activity and reduced adverse effects (in cooperation with the Institute of Inorganic Chemistry and the research cluster “Translational Cancer Therapy Research” (https://tctr.univie.ac.at/)
- Dissection of drug resistance mechanisms and development of circumvention approaches partly in course of the International PhD School Translational Oncology (IPPTO, https://phd-ippto.meduniwien.ac.at/)
Selected publications
- Pirker C, Bilecz A, Grusch M, Mohr T, Heidenreich B, Laszlo V, Stockhammer P, Lötsch-Gojo D, Gojo J, Gabler L, Spiegl-Kreinecker S, Doeme B, Steindl A, Klikovits T, Hoda MA, Jakopovic M, Samarzija M, Mohorcic K, Kern I, Kiesel B, Brcic L, Oberndorfer F, Müllauer L, Klepetko W, Schmidt WM, Kumar R, Hegedus B, Berger W. Telomerase reverse transcriptase promoter mutations identify a genomically defined and highly aggressive human pleural mesothelioma subgroup. Clin Cancer Res. 2020; 26(14):3819-3830. doi: 10.1158/1078-0432.CCR-19-3573.
- Englinger B, Laemmerer A, Moser P, Kallus S, Röhrl C, Pirker C, Baier D, Mohr T, Niederstaetter L, Meier-Menches SM, Gerner C, Gabler L, Gojo J, Timelthaler G, Senkiv J, Jäger W, Kowol CR, Heffeter P, Berger W. Lipid droplet-mediated scavenging as novel intrinsic and adaptive resistance factor against the multikinase inhibitor ponatinib. Int J Cancer. 2020 Sep 15;147(6):1680-1693. doi: 10.1002/ijc.32924.
- 124 I Radiolabeling of a AuIII -NHC Complex for in vivo biodistribution studies. Angew Chem Int Ed Engl. 2020 Jul 7. doi: 10.1002/anie.202008046.
- . Single-cell RNA-seq of pediatric ependymoma reveals prognostic impact of impaired neuronal-glial fate specification. Cancer Cell. 2020 Jul 13;38(1):44-59.e9. doi: 10.1016/j.ccell.2020.06.004.
- Domarco O, Kieler C, Pirker C, Dinhof C, Englinger B, Reisecker JM, Timelthaler G, García MD, Peinador C, Keppler BK, Berger W (corr author), Terenzi A. Subcellular duplex DNA and G-quadruplex interaction profiling of a hexagonal Pt(II) metallacycle. Angew Chem Int Ed Engl. 2019 Jun 11;58(24):8007-8012. doi: 10.1002/anie.201900934.
- Englinger B, Pirker C, Heffeter P, Terenzi A, Kowol CR, Keppler BK, Berger W. Metal Drugs and the Anticancer Immune Response. Chem Rev. 2019 Jan 23;119(2):1519-1624. doi: 10.1021/acs.chemrev. 8b00396.
FG3 Team

Michael Gutmann
F: +43 (0)1 40160-57557
michael.gutmann@meduniwien.ac.at

Assoc. Prof. Priv.-Doz. Mag. Dr. Petra Heffeter, MAS
Center for Cancer Research
Department of Medicine I
Medical University of Vienna
Borschkegasse 8a
1090 Vienna, Austria
T: +43 (0)1 40160-57594
F: +43 (0)1 40160-957502
Research interests
Chemotherapy and therapy with small targeted molecules are two major strategies for therapy of human cancer at the disseminated stage. During the last decades, thousands of compounds have been developed and consequently have improved therapy effectiveness. However, even when using new targeted therapeutics, such therapy is often limited by strong side effects, resistance development and insufficient tumor accumulation.
We focus on the development of several new strategies to overcome these limitations:
- Improvement of anticancer activity by smart drug combinations;
- Development of new targeting strategies to increase drug delivery to the tumor tissue (in cooperation with the Institute of Inorganic Chemistry and the research cluster “Translational Cancer Therapy Research” (https://tctr.univie.ac.at/)
- Investigation on the mechanisms underlying the sensitivity/resistance of cancer cells against new anticancer drugs to define the patient collective which will profit most from chemotherapy in course of the International PhD School Translational Oncology (IPPTO, https://phd-ippto.meduniwien.ac.at/ ).
Selected publications
- Hager S, Pape VFS, Pósa V, Montsch B, Uhlik L, Szakács G, Tóth S, Jabronka N, Keppler BK, Kowol CR, Enyedy EA, Heffeter P. High copper complex stability and slow reduction kinetics as key parameters for improved activity, paraptosis induction and impact on drug-resistant cells of anticancer thiosemicarbazones. Antioxid Redox Signal. 2020 April 25; doi: 10.1089/ars.2019.7854.
- Karmakar S, Poetsch I, Kowol CR, Heffeter P (Corr author), Gibson D. Synthesis and Cytotoxicity of Water-Soluble Dual- and Triple-Action Satraplatin Derivatives: Replacement of Equatorial Chlorides of Satraplatin by Acetates. Inorg Chem. 2019 Dec 16;58(24):16676-16688. doi: 10.1021/acs.inorgchem.9b02796.
- Groza D, Gehrig S, Kudela P, Holcmann M, Pirker C, Dinhof C, Schueffl HH, Sramko M, Hoebart J, Alioglu F, Grusch M, Ogris M, Lubitz W, Keppler BK, Pashkunova-Martic I, Kowol CR, Sibilia M, Berger W, Heffeter P. Bacterial Ghosts as Adjuvant to Oxaliplatin Chemotherapy in Colorectal Carcinomatosis. Oncoimmunology. 2018 Feb 16;7(5):e1424676. doi: 10.1080/2162402X.2018.1424676.
- Hager S, Korbula K, Bielec B, Grusch M, Pirker C, Schosserer M, Liendl L, Lang M, Grillari J, Nowikovsky K, Pape VFS, Mohr T, Szakács G, Keppler BK, Berger W, Kowol CR, Heffeter P. The thiosemicarbazone Me2NNMe2 induces paraptosis by disrupting the ER thiol redox homeostasis based on protein disulfide isomerase inhibition. Cell Death Dis. 2018 Oct 15;9(11):1052. doi: 10.1038/s41419-018-1102-z.
- Schoenhacker-Alte B, Mohr T, Pirker C, Kryeziu K, Kuhn P.S., Buck A, Hofmann T, Gerner C, Hermann G, Koellensperger G, Keppler BK, Berger W, Heffeter P. Sensitivity towards the GRP78 inhibitor KP1339/IT-139 is characterized by apoptosis induction via caspase 8 upon disruption of ER homeostasis. Cancer Letters 2017 Sep 28;404:79-88.
- Mayr J, Heffeter P (Corr. author), Groza D, Galvez L, Koellensperger G, Roller A, Alte B, Haider M, Berger W, Kowol CR, Keppler BK. An albumin-based tumor-targeted oxaliplatin prodrug with distinctly improved anticancer activity in vivo. Chem Sci 2017 Mar 1;8(3):2241-2250.
FG3 Team

Monika Caban
T: +43 (0)1 40160-57620
monika.caban@meduniwien.ac.at

Hemma Schüffl
T: +43 (0)1 40160-57557
hemma.schueffl@meduniwien.ac.at

Isabella Pötsch
T: +43 (0)1 40160-57513
isabella.poetsch@univie.ac.at
isabella.poetsch@meduniwien.ac.at

Univ. Prof. Dr. Gunda Köllensperger
Institute of Analytical Chemistry Chemistry
Senior Scientist
Faculty of Chemistry
University of Vienna
Waehrignerstraße 38
1090 Vienna, Austria
T: +43 (0)1 4277-52303
M: +43 (0)664 60277-52303
gunda.koellensperger@univie.ac.at
Research interests
Gunda Koellensperger addresses analytical metallomic and metabolomic strategies to dissect the key factors for therapy response and resistance with a special focus on single-cell strategies. Her research envisages the development of innovative analytical procedures with emphasis on accurate quantification in omics-type of analysis and cutting-edge elemental mass spectrometry addressing single cell analysis.
Selected publications
- Theiner S, Schweikert A, Van Malderen SJM, Schoeberl A, Neumayer S, Jilma P, Peyrl A, Koellensperger G, Laser Ablation-Inductively Coupled Plasma Time-of-Flight Mass Spectrometry Imaging of Trace Elements at the Single-Cell Level for Clinical Practice, Analytical Chemistry, 2019, DOI 10.1021/acs.analchem.9b00698
- Galvez, L., Rusz, M., Schwaiger-Haber, M., El Abiead, Y., Hermann, G., Jungwirth, U., Berger, W., Keppler, B.K., Jakupec, M.A., Koellensperger, G. Preclinical studies on metal based anticancer drugs as enabled by integrated metallomics and metabolomics (2019) Metallomics, 11 (10), pp. 1716-1728. DOI: 10.1039/c9mt00141g
- Schwaiger M, Schoeny H, El Abiead Y, Hermann G, Rampler E, Koellensperger G, Merging metabolomics and lipidomics into one analytical run, Analyst [2019], 144, 220-229, DOI: 10.1039/C8AN01219A
- Theiner S, Van Malderen SJM, Van Acker T, Legin A, Keppler BK, Vanhaecke F, Koellensperger G. Fast High-Resolution Laser Ablation-Inductively Coupled Plasma Mass Spectrometry Imaging of the Distribution of Platinum-Based Anticancer Compounds in Multicellular Tumor Spheroids, [2017] Analytical Chemistry, 89 (23), pp. 12641-12645. DOI: 10.1021/acs.analchem.7b02681
- Schwaiger M, Rampler E, Hermann G, Miklos W, Berger W, Koellensperger G. Anion-Exchange Chromatography Coupled to High-Resolution Mass Spectrometry: A Powerful Tool for Merging Targeted and Non-targeted Metabolomics. [2017] Analytical Chemistry, 89 (14), pp. 7667-7674. DOI: 10.1021/acs.analchem.7b01624
FG3 Team

Martin Schaier
T: +43 (0)1 4277-52384
martin.schaier@univie.ac.at

Andreas Schweikert
T: +43 (0)1 4277-52384

Sarah Theiner
T: +43 (0)1 4277-52383
sarah.theiner@univie.ac.at

Assoc. Prof. Dr. Christian Kowol
Institute of Inorganic Chemistry
Faculty of Chemistry
University of Vienna
Waehrignerstraße 42
1090 Vienna, Austria
Tel.: +43 (0)1 4277-52611
christian.kowol@univie.ac.at
Research interests
The research of my group focuses on the development of new strategies to overcome severe side effects, resistance development and insufficient tumor accumulation of currently applied anticancer drugs. The strategies include:
- Bioinorganic prodrug development of clinically approved drugs to increase the anticancer activity and prevent the occurrence of severe side effects
- Drug delivery systems like liposomes, polymers and albumin-targeting for tumor-specific accumulation and activation of anticancer drugs
Selected publications
- Bielec, B.; Schueffl, H.; Terenzi, A.; Berger, W.; Heffeter, P.; Keppler, B. K.; Kowol, C. R (corresponding author). Development and biological investigations of hypoxia-sensitive prodrugs of the tyrosine kinase inhibitor crizotinib Bioorg. Chem. 2020, 99, 103778.
- Kastner, A.; Poetsch, I.; Mayr, J.; Burda, J. V.; Roller, A.; Heffeter, P.; Keppler, B. K.; Kowol, C. R (corresponding author). A dogma in doubt: aquation of equatorial ligands of Pt(IV) complexes under physiological conditions Angew. Chem. Int. Ed. 2019, 58, 22, 7464-7469.
- Heffeter, P.; Pape, V. F. S.; Enyedy, E. A.; Keppler, B. K.; Szakacs, G.; Kowol, C. R (corresponding author). Anticancer thiosemicarbazones: chemical properties, interaction with iron metabolism, and resistance development. Antioxid. Redox. Sign. 2019, 30, 1062-1082.
- Englinger, B.; Pirker, C.; Heffeter, P.; Terenzi, A.; Kowol, C.R.; Keppler, B. K.; Berger, W. Metal drugs and the anticancer immune response. Chem. Rev. 2019, 119, 1519-1624.
- Kallus, S.; Englinger, B.; Senkiv, J.; Laemmerer, A.; Heffeter, P.; Berger, W.; Kowol, C. R. (corresponding author); Keppler, B. K. Nanoformulations of Anticancer FGFR Inhibitors with Improved Therapeutic Index. Nanomed. Nanotechnol. 2018, 14, 2632-2643.
- Mayr, J.; Heffeter, P.; Groza, D.; Galvez, L.; Koellensperger, G.; Roller, A.; Alte, B.; Haider, M.; Berger, W.; Kowol, C. R. (corresponding author); Keppler, B. K. An albumin-based tumor-targeted oxaliplatin prodrug with distinctly improved anticancer activity in vivo. Chem. Sci. 2017, 8, 2241–2250.
FG3 Team

Alexander Kastner, MSc
T: +43 (0)1 4277-52616
alexander.kastner@univie.ac.at

Philipp Fronik
T: +43 (0)1 4277-52616
philipp.fronik@univie.ac.at

Dr. Evelyn Rampler
Institute of Analytical Chemistry Chemistry
Senior Scientist
Faculty of Chemistry
University of Vienna
Waehrignerstraße 38
1090 Vienna, Austria
T: +43 (0)1 4277-52381
M: +43 (0)664 60277-52381
evelyn.rampler@univie.ac.at
Research interests
My group focuses on the development of analytical workflows for the analysis of lipids and glycolipids. Within the FWF research group on the action of metal anti-cancer drugs in the tumor microenvironment, we investigate how tumor-specifically activated drugs impact on the lipidome of cancer cells. For this purpose, we will extend our existing analytical toolbox based on liquid chromatography and high resolution mass spectrometry for lipid profiling in tumor cell compartments such as lipid droplets. Our final goal is to understand lipid-based communication with the tumor microenvironment.
Selected publications
- M. Rusz, E. Rampler, B.K. Keppler, M. A. Jakupec, G. Koellensperger, Single spheroid metabolomics: Optimizing sample preparation of three-dimensional multicellular tumor spheroids, Metabolites, 2019, 9, 304, doi: 10.3390/metabo9120304
- E. Rampler, D. Egger, H. Schoeny, M. Rusz, M.P. Pacheco, G. Marino, C. Kasper, T. Naegele, G. Koellensperger, The Power of LC-MS Based Multiomics: Exploring Adipogenic Differentiation of Human Mesenchymal Stem/Stromal Cells, Molecules, 2019, 24, 3615, doi:10.3390/molecules24193615
- E. Rampler, A. Criscuolo, M. Zeller, Y. El Abiead, H. Schoeny, G. Hermann, E. Sokol, K. Cook, D. A. Peake, B. Delanghe, G. Koellensperger, Analytical Chemistry, 2018, 90, 11, 6494-6501, A novel lipidomics workflow for improved human plasma identification and quantification using RPLC-MSn methods and isotope dilution strategies. Invitation for cover suggestion
doi: 10.1021/acs.analchem.7b05382 - E. Rampler*, H. Schoeny, B. M. Mitic, Y. El Abiead, M. Schwaiger, G. Köllensperger*, Analyst, 2018, 143, 1250-1258, Simultaneous non-polar and polar lipid analysis by on-line combination of HILIC, RP and high resolution MS.doi: 10.1039/c7an01984j.
- E. Rampler, C. Coman, G. Hermann, A. Sickmann, R. Ahrends, G. Koellensperger, Analyst, 2017, 142, 1891-1899, LILY-Lipidome Isotope Labeling of Yeast: In vivo synthesis of 13C labeled reference lipids for quantification by mass spectrometry. Open access. HOT paper
doi: 10.1039/c7an00107j
FG3 Team

Katharina Hohenwallner
T: +43 (0)1 4277-52380
katharina.hohenwallner@univie.ac.at
Publications
- Mathuber, M. et al. Development of a cobalt(III)-based ponatinib prodrug system. Inorg. Chem. Front. 2021, 8, 2468–2485. doi.org/10.1039/D1QI00211B
- Schueffl, H. et al. Albumin-targeting of an oxaliplatin-releasing platinum(iv) prodrug results in pronounced anticancer activity due to endocytotic drug uptake in vivo. Chem Sci. 2021 Aug 26;12(38):12587-12599. doi: 10.1039/d1sc03311e.
- Fronik, P. et al. A platinum(IV) prodrug strategy to overcome glutathione-based oxaliplatin resistance. Comm. Chem. 2022, 5, 46 . doi.org/10.1038/s42004-022-00661-z
- Hohenwallner K et al. JACS Au. 2022 Oct 25;2(11):2466-2480. doi: 10.1021/jacsau.2c00230. eCollection 2022 Nov 28.
- Mendrina, T. et al. Influence of the Fatty Acid Metabolism on the Mode of Action of a Cisplatin(IV) Complex with Phenylbutyrate as Axial Ligands. Pharmaceutics 2023, 15, 677 . doi: 10.3390/pharmaceutics15020677.
- Kastner, A et al. Tumor-targeted dual-action NSAID-platinum(IV) anticancer prodrugs. Inorg. Chem. Frontiers 2023, 10, 4126-1438. doi.org/10.1039/D3QI00968H.
- Schaier, M. et al. Human Serum Albumin as a Copper Source for Anticancer Thiosemicarbazones. Metallomics 2023, 15, mfad046
- Caban, M. et al. A novel EGFR inhibitor acts as potent tool for hypoxia-activated prodrug systems and exerts strong synergistic activity with VEGFR inhibition in vitro and in vivo. Cancer Lett. 2023, 565, 216237. doi: 10.1016/j.canlet.2023.216237.
- Kastner et. Et al. Insertion of (bioactive) equatorial ligands into platinum(IV) complexes. Angew. Chem. 2023, 62, e202311468. https://doi.org/10.1002/anie.202311468.
- Van Acker, T. et al. Inductively coupled plasma mass spectrometry (2023) Nature Reviews Methods Primers, 3 (1), art. no. 52, DOI: 10.1038/s43586-023-00235-w
- Baier, D. et al. The Lipid Metabolism as Target and Modulator of BOLD-100 Anticancer Activity: Crosstalk with Histone Acetylation (2023) Advanced Science, 10 (32), art. no. 2301939. DOI: 10.1002/advs.202301939
- Metarapi, D. et al. Semiquantitative Analysis for High-Speed Mapping Applications of Biological Samples Using LA-ICP-TOFMS (2023) Analytical Chemistry, 95 (19), pp. 7804-7812. DOI: 10.1021/acs.analchem.3c01439
- Babu, T. et al. Oral Anticancer Heterobimetallic PtIV−AuI Complexes Show High In Vivo Activity and Low Toxicity (2023) Angewandte Chemie - International Edition, 62 (10), art. no. e202217233 DOI: 10.1002/anie.202217233
- Schaier, M. et al. Multiparametric Tissue Characterization Utilizing the Cellular Metallome and Immuno-Mass Spectrometry Imaging (2023) JACS Au, 3 (2), pp. 419-428. DOI: 10.1021/jacsau.2c00571
- Kastner, A. et al. Stepwise Optimization of Tumor-Targeted Dual-Action Platinum(IV)-Gemcitabine Prodrugs. Inorg. Chem. Frontiers 2024, 11, 534-548 (IF = 7.0), DOI: 10.1039/D3QI02032K.